Reading: Photosynthesis
Douglas Wilkin, Ph.D.
Barbara Akre
http://www.ck12.org/saythanks
• Summarize the process of photosynthesis and write out the overall chemical equation for photosynthesis.
• Identify reactants, necessary conditions, and products in the chemical equation for photosynthesis.
• Understand that hundreds of years of scientific exploration have contributed to our understanding of photosynthesis.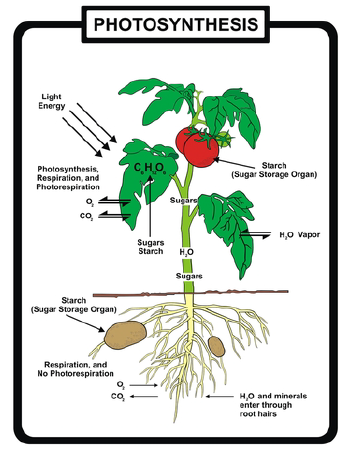
What is the most common biochemical reaction ever?
Well, it may or may not be this one. Every split second that sunlight strikes a plant’s leaf, the process of photosynthesis begins. That’s on every leaf, on every plant, including all the blades of grass. All over the world.
Photosynthesis: The Most Important Chemical Reaction for Life on Earth
What do pizza, campfires, dolphins, automobiles, and glaciers have in common? In the following section, you’ll learn that all five rely on photosynthesis, some in more ways than one. Photosynthesis is often considered the most important chemical reaction for life on earth. Let’s delve into how this process works and why we are so indebted to it.
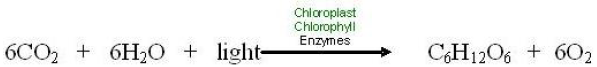
Photosynthesis involves a complex series of chemical reactions, each of which convert one substance to another. These reactions taken as a whole can be summarized in a single symbolic representation –as shown in the chemical equation below.
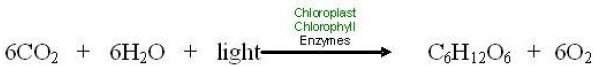
We can substitute words for the chemical symbols. Then the equation appears as below.
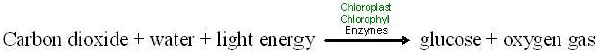
Like all chemical equations, this equation for photosynthesis shows reactants connected by plus signs on the left and products, also connected by plus signs, on the right. An arrow indicating the process or chemical change leads from the reactants to the products, and conditions necessary for the chemical reaction are written above the arrow. Note that the same kinds of atoms, and number of atoms, are found on both sides of the equation, but the kinds of compounds they form change
You use chemical reactions every time you cook or bake. You add together ingredients (the reactants), place them in specific conditions (often heat), and enjoy the results (the products). A recipe for chocolate chip cookies written in chemical equation form is shown below.

Compare this familiar recipe to photosynthesis below.
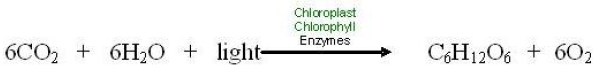
The equation shows that the “ingredients” for photosynthesis are carbon dioxide, water, and light energy. Plants, algae, and photosynthetic bacteria take in light from the sun, molecules of carbon dioxide from the air, and water molecules from their environment and combine these reactants to produce food (glucose).
Of course, light, carbon dioxide, and water mix in the air even without plants. But they do not chemically change to make food without very specific necessary conditions which are found only in the cells of photosynthetic organisms. Necessary conditions include:
1. enzymes - proteins which speed up chemical reactions
2. chlorophyll - a pigment within plant cells which absorbs light
3. chloroplasts - organelles whose membranes embed chlorophyll, accessory pigments, and
enzymes in patterns which maximize photosynthesis
Within plant cells or algal cells, chloroplasts organize the enzymes, chlorophyll, and accessory pigment molecules necessary for photosynthesis.
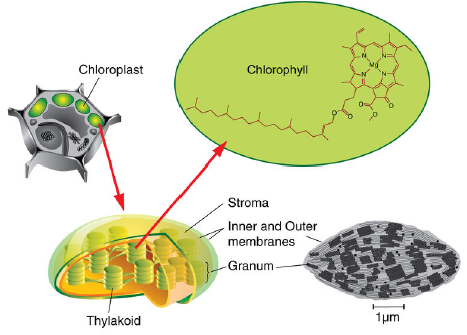
FIGURE 1.1
Within plant cells or algal cells, chloroplasts organize the enzymes, chlorophyll, and accessory pigment molecules necessary for photosynthesis.
When the reactants meet inside chloroplasts, or the very similar cells of blue-green bacteria, chemical reactions combine them to form two products: energy-rich glucose molecules and molecules of oxygen gas. Photosynthetic organisms store the glucose (usually as starch) and release the oxygen gas into the atmosphere as waste.
Let’s review the chemical equation for photosynthesis once more, this time at the level of atoms as in the equation below.
Look closely at its primary purpose: storing energy in the chemical bonds of food molecules. The source of energy for food is sunlight energy. The source of carbon atoms for the food molecules is carbon dioxide from the air, and the source of hydrogen atoms is water. Inside the cells of plants, algae, and photosynthetic bacteria, chlorophyll, and enzymes use the light energy to rearrange the atoms of the reactants to form the products, molecules of glucose and oxygen gas. Light energy is thus transformed into chemical energy, stored in the bonds which bind six atoms each of carbon and oxygen to twelve atoms of hydrogen –forming a molecule of glucose. This energy rich carbohydrate molecule becomes food for the plants, algae, and bacteria themselves as well as for the heterotrophs which feed on them.
One last detail: why do “6”s precede the CO2, H2O, and O2? Look carefully, and you will see that this “balances”
the equation: the numbers of each kind of atom on each side of the arrow are equal. Six molecules each of CO2 and H2O make 1 molecule of glucose and 6 molecules of oxygen gas.
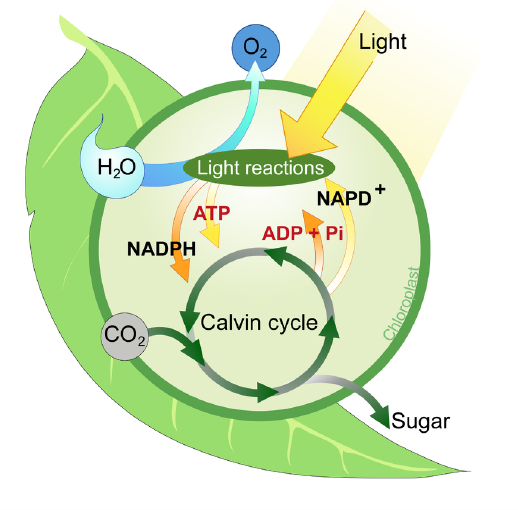
FIGURE 1.2
The two stages of photosynthesis are the light reactions and the Calvin cycle. Do you see how the two stages are related?
Historical Perspective
Life requires photosynthesis for fuel and for the oxygen to burn that fuel. Since the Industrial Revolution (late 18th and early 19th centuries), we humans have relied on products of ancient photosynthesis for enormous quantities of fossil fuel energy. And, knowingly or not, we have also benefited from photosynthesis to remove the carbon dioxide produced when we burn those fuels. So it may not surprise you that biologists have studied this critical process in great detail.
Although photosynthesis may seem straightforward in this form, such simplicity is deceiving for two reasons. First, the photosynthesis equation summarizes dozens of individual chemical reactions involving many intermediate compounds. And second, just discovering major players like CO2 and O2 was challenging, because our ordinary senses cannot detect these molecules in “thin air!”
How do we know that the chemical reaction in photosynthesis really happens? Two famous historical experiments help us begin to understand this process. In the 17th century, people who thought about it at all assumed that plants get their food from the soil. Many people, encouraged by sellers of “plant food,” still do. In 1638, Jan Baptist Van Helmont planted a 5 pound willow tree, like the one shown in the Figure 1.3, in a 200 pound tub of soil. After 5 years of watering the plant, he weighed both again. The willow had gained over 160 pounds, but the soil had lost only 2 ounces. Van Helmont concluded that plants do not get their materials from soil, and inferred that they grow using materials from water (which he did not measure). As you know now, he was half right. Although soil provides important nutrients to plants, it supplies neither the energy nor the vast majority of the materials to build the plant. We must excuse him, because no one in the 17th century knew that carbon atoms form the basis of life, or that they float around in air in the form of carbon dioxide.
In the late 1770s, minister and natural philosopher Joseph Priestley burned a candle in a jar of air and observed that the candle burned out long before it ran out of wax. A similar experiment with a mouse resulted in the mouse’s death. Priestley suggested that animals, like candles, “injure” the air. Adding a mint plant, as shown in Figure 1.4, however, “restored” the air which had been “injured” by the mouse or the candle. Only later, after many chemistry experiments, did Priestley publish his discovery of “dephlogisticated air.” But in his studies of mice, plants, and candles, he had shown that plants produce, and animals consume, oxygen gas.
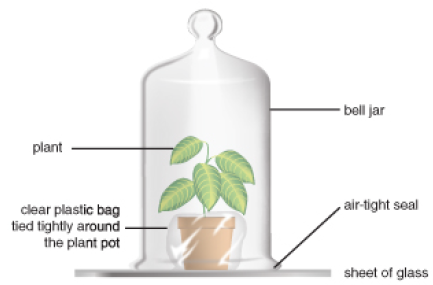
FIGURE 1.4
Joseph Priestly’s bell jar experiment.
During the 20th century, scientists learned that photosynthesis involves much more than just the three reactants, the three necessary conditions, and the two products shown in the equation. Using powerful microscopes, scientists narrowed the process to one type of organelle within the plant –the chloroplast.
Vocabulary
• chlorophyll: The primary pigment of photosynthesis; found in the thylakoid membranes of chloroplasts.
• chloroplast: The organelle of photosynthesis; site of photosynthesis.
• enzyme: Chemical, usually a protein, that speeds up chemical reactions in organisms; a biological catalyst.
Summary
• The photosynthesis chemical equation states that the reactants (carbon dioxide, water and sunlight), yield two products, glucose and oxygen gas.
• The single chemical equation represents the overall process of photosynthesis. It also summarizes many individual chemical reactions that were understood only after hundreds of years of scientific exploration.
• Chloroplasts are the organelles within plant and algal cells that organize enzymes and pigments so that the chemical reactions proceed efficiently.
• Chlorophyll is a pigment that absorbs sunlight energy.
Review
1. Using symbols, write the overall chemical equation for photosynthesis, labeling the reactants, necessary conditions, and products.
2. Summarize Jan Van Helmont’s willow tree experiment. State his conclusion and the inference he made after his experiment, and explain how his data supports each. Finally, relate his findings to what we know today about the overall process of photosynthesis.
3. Using the overall equation for photosynthesis, explain which components relate to J.B. Priestley’s observation that “Plants restore the air that animals injure.”
