Reading: No Health Without Mental Health
Global Mental Health 1
No health without mental health
Martin Prince, Vikram Patel, Shekhar Saxena, Mario Maj, Joanna Maselko, Michael R Phillips, Atif Rahman
About 14% of the global burden of disease has been attributed to neuropsychiatric disorders, mostly due to the chronically disabling nature of depression and other common mental disorders, alcohol-use and substance-use disorders, and psychoses. Such estimates have drawn attention to the importance of mental disorders for public health. However, because they stress the separate contributions of mental and physical disorders to disability and mortality, they might have entrenched the alienation of mental health from mainstream efforts to improve health and reduce poverty. The burden of mental disorders is likely to have been underestimated because of inadequate appreciation of the connectedness between mental illness and other health conditions. Because these interactions are protean, there can be no health without mental health. Mental disorders increase risk for communicable and non-communicable diseases, and contribute to unintentional and intentional injury. Conversely, many health conditions increase the risk for mental disorder, and comorbidity complicates help-seeking, diagnosis, and treatment, and influences prognosis. Health services are not provided equitably to people with mental disorders, and the quality of care for both mental and physical health conditions for these people could be improved. We need to develop and evaluate psychosocial interventions that can be integrated into management of communicable and non-communicable diseases. Health-care systems should be strengthened to improve delivery of mental health care, by focusing on existing programmes and activities, such as those which address the prevention and treatment of HIV, tuberculosis, and malaria; gender-based violence; antenatal care; integrated management of childhood illnesses and child nutrition; and innovative management of chronic disease. An explicit mental health budget might need to be allocated for such activities. Mental health affects progress towards the achievement of several Millennium Development Goals, such as promotion of gender equality and empowerment of women, reduction of child mortality, improvement of maternal health, and reversal of the spread of HIV/AIDS. Mental health awareness needs to be integrated into all aspects of health and social policy, health-system planning, and delivery of primary and secondary general health care.
Lancet 2007; 370: 859–77
Published Online September 4, 2007 DOI:10.1016/S0140- 6736(07)61238-0
This is the first in a Series of six papers about global mental health
See Comment page 806 and page 810
See Perspectives page 821
King’s College London, Centre for Public Mental Health, Health Service and Population Research Department, Institute of Psychiatry, London, UK
(Prof M Prince MD); Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK and Sangath, Goa, India
(Prof V Patel PhD); Department of Mental Health and Substance Abuse, World Health
Organization, Geneva,
Introduction
The WHO proposition that there can be “no health without mental health”1 has also been endorsed by the Pan American Health Organisation, the EU Council of Ministers, the World Federation of Mental Health, and the UK Royal College of Psychiatrists. What is the substance of this slogan?
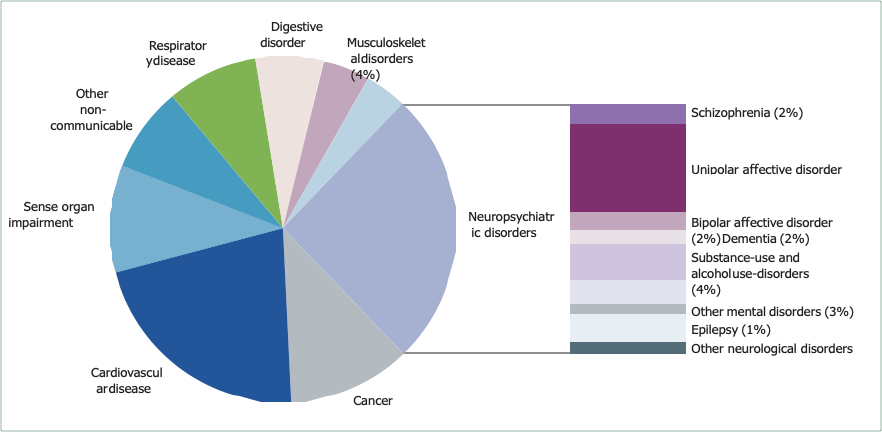
Mental disorders make a substantial independent contribution to the burden of disease worldwide (panel 1).2 WHO’s 2005 estimates of the global burden of disease provide evidence on the relative effect of health problems worldwide.3,4 Non-communicable diseases are rapidly becoming the dominant causes of ill health in all developing regions except sub-Saharan Africa (table 1).4 The Global Burden of Disease report has revealed the scale of the contribution of mental disorders, by use of an integrated measure of disease burden—the disability-adjusted life-year, which is the sum of years lived with disability and years of life lost.4 The report showed that neuropsychiatric conditions account for up to a quarter of all disability-adjusted life-years, and up to a third of those attributed to non-communicable diseases, although the size of this contribution varies between countries according to income level (table 1).4 The neuropsychiatric conditions that contribute the most disability-adjusted life-years are mental disorders, especially unipolar and bipolar affective disorders, substance-use and alcohol-use disorders, schizophrenia,
and dementia. Neurological disorders (such as migraine, epilepsy, Parkinson’s disease, and multiple sclerosis) make a smaller but still significant contribution. Of the non-communicable diseases, neuropsychiatric conditions contribute the most to overall burden (figure 1 and table 1),4 more than either cardiovascular disease or cancer.
Switzerland (S Saxena MD) Department of Psychiatry, University of Naples, Naples, Italy (Prof M Maj PhD) Department of Public Health, Temple University College of Health Professions, Philadelphia, Pennsylvania, USA (J Maselko ScD); Beijing Suicide Research and Prevention Centre, Beijing Hui Long Guan Hospital, Beijing, China and Departments of Psychiatry and Epidemiology, Columbia University, New York, USA;
(Prof M R Phillips MD) Division
of Psychiatry, University of Manchester, Manchester UK and Institute of Psychiatry,
Rawalpindi, Pakistan
(A Rahman PhD)
Correspondence to:
Prof Martin Prince, Centre for Public Mental Health, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK m.prince@iop.kcl.ac.uk
Despite these new insights, ten years after the first WHO report on the global burden of disease, mental health remains a low priority in most low-income and middle- income countries. Developing countries tend to prioritise the control and eradication of infectious diseases and reproductive, maternal, and child health, whereas devel- oped countries prioritise non-communicable diseases that cause early death (such as cancer and heart disease) above those that cause years lived-with-disability (such as mental disorders, dementia, and stroke). If mental disorders are regarded as a distinct health domain, with separate services and budgets, then investment in mental health is perceived to have an unaffordable opportunity cost.
Our first aim is to critically appraise the way that the burden of disability and premature mortality is apportioned, in WHO’s estimates of global burden of disease, between underlying conditions within groups of disorder, and, specifically, to assess whether these estimates account for the full contribution of mental disorder to mortality and disability. Our second aim is to review available evidence for interactions between mental disorders and other health conditions (such as medically
unexplained somatic symptoms, communicable diseases, maternal and perinatal conditions, non-communicable diseases, and injuries). Our third aim is to discuss the implications of these links for the future orientation of health policies, health systems, and services.
Contributions of mental disorders to disability and mortality
Mental disorders are an important cause of long-term disability and dependency. WHO’s 2005 report attributed 31∙7% of all years lived-with-disability to neuropsychiatric conditions: the five major contributors to this total were unipolar depression (11∙8%), alcohol-use disorder (3∙3%), schizophrenia (2∙8%), bipolar depression (2∙4%), and dementia (1∙6%).4 However, the interaction between mental disorder and disability is more complex and extensive than the WHO report suggests. Depression predicts the onset and progression of both physical and social disability.5,6 Conversely, disability is an important prospective risk factor for depression in older adults,7–12 and mediates most of the effects of specific physical health conditions in this group.10,13–15 Social support is an effect modifier.10,11,16 The population-attributable fraction (which is the proportion of cases of disability that would not have occurred in the absence of mental disorders) could be as high as 0∙69,10 which suggests that failing health and consequent disability could be the most important contributory cause for late-life depression. Two studies suggest that disability is an equally powerful, although less prevalent, prospective risk factor for depression in young people.17,18
Mental disorders also contribute to mortality. According to WHO’s 2005 estimates, neuropsychiatric disorders account for 1∙2 million deaths every year and 1∙4% of all years-of-life lost; most of these are caused by dementia, Parkinson’s disease, and epilepsy.4 Only 40 000 deaths were attributed to mental disorders (mainly unipolar and bipolar depression, schizophrenia, and post-traumatic stress disorder) and 182 000 to use of drugs and alcohol.4 These numbers are almost certainly underestimated, since the report attributes death by suicide to intentional injury.4 Every year, about 800 000 people commit suicide, 86% of whom are in low-income and middle-income countries, and more than half of whom are aged between
15 and 44 years. Even these figures might be under- estimated, since official statistics in low-income and middle-income countries are not reliable. For example, studies in south India that used surveillance with validated verbal autopsy showed that rates of suicide were ten times greater than the official national estimates;19,20 that suicide was the leading cause of death in 10–19 year olds; and that suicides accounted for a quarter of all deaths in boys and up to three-quarters of all deaths in young women.19 A systematic review of psychological autopsy case-control studies identified mental disorders (depression, schizophrenia and other psychoses, and alcohol-use and substance-use disorders) as important proximal risk factors for suicide, with a median prevalence of mental
|
|
|
2005 |
|
|
|
Projected for 2030 |
|
|||
|
|
World |
High-income |
Middle-income |
Low-income |
World |
High-income |
Middle-income |
Low-income |
|
|
|
|
|
countries |
countries |
countries |
|
countries |
countries |
countries |
|
|
|
Total DALYs |
1 483 060 000 |
119 361 000 |
492 549 000 |
871 141 000 |
1650 629 000 |
118 309 000 |
528 066 000 |
1 004 236 000 |
|
|
|
I Communicable, |
572 292 000 |
6 647 000 |
99 696 000 |
465 948 000 |
494 384 000 |
4 060 000 |
79 623 000 |
410 698 000 |
|
|
|
maternal, perinatal, and nutritional conditions |
(38·6%) |
(5·6%) |
(20·2%) |
(53·5%) |
(30·0%) |
(3·4%) |
(15·1%) |
(40·9%) |
|
|
|
II Non-communicable |
725 506 000 |
102 311 000 |
318 415 000 |
304 773 000 |
938 468 000 |
105 716 000 |
380 324 000 |
452 416 000 |
|
|
|
diseases |
(48·9%) |
(85·7%) |
(64·7%) |
(35%) |
(56·9%) |
(89·4%) |
(72%) |
(45·1%) |
|
|
|
Neuropsychiatric |
199 606 000 |
32 717 000 |
87 398 000 |
79 490 000 |
237 962 000 |
34 798 000 |
92 590 000 |
110 571 000 |
|
|
|
conditions |
(13·5%) |
(27·4%) |
(17·7%) |
(9·1%) |
(14·4%) |
(29·4%) |
(17·5%) |
(11·0%) |
|
|
|
|
(27·5%)* |
(32·0%)* |
(27·5%)* |
(26·1%)* |
(25·4%)* |
(32·9%)* |
(24·3%)* |
(24·4%)* |
|
|
|
III Injuries |
185 262 000 |
10 403 000 |
74 439 000 |
100 420 000 |
217 777 000 |
8 533 000 |
68 120 000 |
141 122 000 |
|
|
|
|
|
(12·5%) |
(8·7%) |
(15·1%) |
(11·5%) |
(13·2%) |
(7·2%) |
(12·9%) |
(14·1%) |
|
|
DALYs=disability-adjusted life-years. Data are DALYs (proportion of total DALYs), unless otherwise specified. *Proportion of non-communicable disease DALYs caused by neuropsychiatric conditions. Table 1: Contribution by different health conditions to disability-adjusted life-years, by income level of countries |
|
|||||||||
Figure 1: Contribution by different non-communicable diseases to disability-adjusted life-years worldwide in 2005
Data adapted from WHO, with permission.3
disorder of 91% in suicide completers, and a population- attributable fraction of 47–74%.21 Findings from psychological autopsy studies in India and China were similar.22,23 Therefore, prevention, identification, and appropriate management of mental health problems is an important element of suicide prevention.
Mental disorder is independently associated with a substantial excess in all-cause mortality risk. Most studies have focused on associations with depression: a meta-analysis of 15 population-based studies reported that depression diagnosis was linked with subsequent all-cause mortality, and yielded a pooled odds ratio (OR) of 1∙7 (95% CI 1∙5–2∙0).24 Several studies report that this association is mediated partly through disability,25 but not through cardiovascular disease, cardiovascular risk factors, or antidepressant use.26 Increased all-cause mortality, excluding suicides, has also been reported for schizophrenia
(relative risk [RR] 2∙59, 95% CI 2∙55–2∙63),27 bipolar disorder (standardised mortality ratio [SMR] 1∙9 for men and 2∙1 for women),28 and dementia (RR 2∙63, 95% CI 2∙17–3∙21).29 In a record linkage study of mental health service users from western Australia,30 mortality from ischaemic heart disease was linked with most mental disorders, especially dementia and schizophrenia and other psychoses, although rates of admission for ischaemic heart disease were similar. People with schizophrenia (RR for men 0∙31 [95% CI 0∙21–0∙45] and for women 0∙34 [0∙18–0∙64]) and people with dementia (RR for men 0∙14 [0∙07–0∙26] and for women 0∙53 [0∙16–1∙74]) were much less likely to undergo revascularisation procedures such as coronary artery bypass grafting. In the general population, between 1980 and 1998, ischaemic heart disease mortality fell by 34% in men and 13% in women, but in users of mental health services the rate was stable in men and had
increased by 40% in women. Although evidence from low income countries is scarce, a large population-based study in Ethiopia indicated very high mortality rates for major depression (SMR 3∙55, 95% CI 1∙97–6∙39)31 and for schizophrenia (nearly 5% per year).32 The association between alcohol use and mortality is complex, with a U-shaped association, and different effects according to cause of death; nevertheless, in the UK 8∙5% of years-of-life lost to age 65 in men and 4∙0% in women have been attributed to drinking more than the recommended alcohol limits.33 In Russia, alcohol-related mortality contributed to substantial fluctuations in the overall mortality rate in the 1990s.34
Mental disorders interact with other health conditions
Medically unexplained somatic symptoms
Typically, at least a third of all somatic symptoms remain medically unexplained, both in the general population35 and in general medical-care settings.36 Common medically unexplained symptoms include pain, fatigue, and dizziness. Syndromes that represent characteristic organ- specific groups of medically unexplained symptoms have also been defined: irritable bowel syndrome, fibromyalgia, chronic-fatigue syndrome, chronic pelvic pain, temporo- mandibular joint dysfunction, and sexual-discharge syndromes. Medically unexplained somatic symptoms37 and syndromes38 are strongly associated with common mental disorders; however, at least a third of those with somatisation have no comorbid mental disorder.39,40 About 15% of patients seen in primary care have somatisation, which is defined as medically unexplained somatic symptoms coupled with psychological distress and help-seeking behaviour.40,41 Somatisation is independently associated with poor health-related quality of life40–42 and greatly increased use of health care,39 after controlling for comorbid mental disorder. In the USA, somatisation is estimated to contribute US$256 billion to health-care costs every year.39 Evidence from randomised controlled trials supports the effectiveness of specific intervention strategies such as structured treatment recom- mendations,43,44 antidepressant medication,45 and cogni- tive behaviour therapy46,47 for reduction of somatic symptoms and health-care use. Health-care costs can be reduced by as much as a third.43 A pilot trial of cognitive behavioural therapy for medically unexplained symptoms in Sri Lankan primary care (the only published trial from a developing country) also showed that treatment was associated with significant reductions in medically unexplained symptoms, visits, and distress.48
Non-communicable diseases
Aside from neuropsychiatric disorders, the main contributors to disability and mortality from non- communicable disease are cardiovascular disease and cancer. Coronary heart disease and stroke account for 21% of disability-adjusted life-years in this group, and
cancer for 12% (figure 1).4 Endocrine disorders (primarily diabetes) account for 3·7% of the disability-adjusted life-years attributed to non-communicable disease, and this proportion is predicted to rise sharply to 5∙4% by 2030, with much of the increase in low-income and middle-income countries.4 Non-communicable diseases are a global challenge: they are the leading cause of death in all world regions other than sub-Saharan Africa, with 80% of deaths in low-income and middle-income countries.49
Cardiovascular disease
A systematic review of evidence from population- based research reported moderate to strong prospective associations between depression (15/22 studies), anxiety (four of eight studies), and coronary heart disease.50,51 The outcomes studied included angina and non-fatal and fatal myocardial infarction.50,51 Population-based cohort studies also show that depression is an independent risk factor for non-fatal52–54 and fatal55 stroke. Follow-up periods in many of these studies were longer than ten years, which renders depression induced by preclinical cardiovascular disease an unlikely explanation. The effects were largely independent of risk factors for cardiovascular disease, since most of the cited studies comprehensively controlled for such factors.
The scarcity of evidence for risk mediation is surprising since mental health is strongly associated with cardiovascular risk exposures. Obesity, in a nationally representative survey in the USA, was associated with significant increases in lifetime diagnoses of major depression, bipolar disorder, and panic disorder or agoraphobia.56 Smoking, in population-based studies, is consistently shown to be associated with depressive and anxiety disorders (OR 1∙5–2∙0),57–59 and with schizophrenia (OR 5∙9).60 These associations might be bidirectional; prospective studies of young people indicate both that affective disorders and alcohol-use disorders could predict adoption of a daily smoking habit,61,62 and that tobacco use can be associated with the onset of common mental disorder.63 Findings from prospective population-based studies conflict as to whether mental disorders predict failure to quit smoking in those with the habit.59,64 In a study with a 7–16 year follow-up of participants,65 incident hypertension was independently predicted by both high depression scores (OR 1∙8, 95% CI 1∙2–2∙8) and anxiety scores (1∙8, 1∙3–2∙5) at baseline, after controlling for age, sex, education, smoking, body-mass index, alcohol use, history of diabetes or cardiovascular disease, and baseline systolic blood pressure.
The incidence of depression increases after myocardial infarction, to 15–30% for major depression, mostly in the first month after the event.66 Systematic reviews of prognostic studies report that comorbid depression is a consistent predictor of adverse outcomes (including recurrent coronary heart disease events, mortality from
coronary heart disease, and all-cause mortality) after non-fatal myocardial infarction, after controlling for disease severity and treatment-related factors.50,51 Poor prognosis might be mediated partly by poor adherence by patients with depression to behaviour and lifestyle changes intended to reduce the risk of subsequent cardiac events.67 The evidence for anxiety as a prognostic factor is less strong.51,68,69 In a study based on the Maastricht stroke registry, the cumulative 1-year incidence of major depression was 23∙3%.70 Two population-based incidence studies71,72 support a strong association between recent incident stroke and subsequent onset of depression, independent of disability. Depression after stroke is associated with poor functional outcomes73,74 and with a 3∙4 times higher mortality over 10 years, after adjusting for baseline severity and type of stroke.75
A Cochrane review of 36 trials of psychological interventions after myocardial infarction (18 of which focused on stress management) did not report an effect on total or cardiac mortality, but did show small reductions in anxiety and depression in patients with coronary heart disease.76 Few interventions have specifically targeted affective disorder. Antidepressants (selective serotonin-reuptake-inhibitors [SSRI]) have been shown to be safe and moderately effective treatments for depression after myocardial infarction.77,78 A large trial of stepped-care cognitive behavioural therapy and SSRIs for depression and perception of low social support after myocardial infarction reported that the intervention was associated with significant improvement in mood and social support but not with improvement in event-free or overall survival.79 Therefore, more intensive and flexible patient-specific interventions have been advocated.80
The evidence base for the effectiveness of antidepressants after stroke is weak. A Cochrane review of antidepressants as a preventive intervention reported no effect either on incident depression, or on reduction of disability or mortality.81 Another Cochrane review on pharmacological interventions for depression after stroke reported a reduction in symptoms, but not remission of diagnosable depression.82 Stroke recovery was not improved by pharmacological interventions.82 One trial subsequently published, with a 9-year follow-up, did show a sustained reduction in mortality after stroke, associated with antidepressant treatment.83
Diabetes
Two US population cohort studies suggested that depression increases the risk for onset of type 2 diabetes, controlling for demographic, metabolic, and lifestyle factors;84,85 however, another large cohort study did not support this finding.86 The prospective associations might yet be explained by undetected diabetes leading to depression, or by help-seeking for depression leading to detection of diabetes. The evidence for comorbidity between mental disorder and diabetes is much stronger. The prevalence of diabetes in people with schizophrenia
has consistently been shown to be about 15%, compared with a typical community prevalence of 2–3%.87 Much of this difference is probably explained by lifestyle factors, and some by the metabolic effects of typical and atypical antipsychotic medication.87 Abnormalities of glucose regulation were noted in people with schizophrenia before the use of antipsychotic medication,87 and independent of treatment in the modern era.88,89 The increased frequency of a family history of diabetes in people with schizophrenia87 also suggests an underlying mechanism specific to the disease. A meta-analysis of the association between depression and diabetes identified 20 controlled studies (of which 11 were population-based) with an OR for the association between the two conditions of 2∙0 (95% CI 1∙8–2∙2); this ratio did not vary by type of diabetes, method for assessment of depression, or study design.90 Data on comorbid anxiety and diabetes were sparser, with only five controlled studies, one of which was population-based; the mean rate of generalised anxiety disorder in the clinical samples was 13∙5%, which is much higher than the 3–4% typically seen in community studies.91 Comorbidity between diabetes and common mental disorder is important because of the implications for chronic disease management, and the effect on diabetic outcomes.
People with schizophrenia show poor adherence to oral hypoglycaemic therapy.92 Adherence to recommendations for diet93,94 and exercise,94 and to oral hypoglycaemic medication93,94 is low in diabetics with depression. In one study, however, attendance for screening by medical services to prevent complications was not affected by mood.94 Similar effects on adherence were noted for alcohol consumption in diabetics from ethnic minorities in Los Angeles.95 Poor mental health seems to have the greatest effects on patient-initiated behaviours that are difficult to maintain.94 The quality of diabetic care received by those with and without mental disorders, including serious mental illness, seems to be similar for most indicators,96,97 with the possible exception of those with substance-use disorders.97 Even so, meta-analyses suggest that both depression98 and anxiety99 are associated with poor glycaemic control. These cross-sectional associations are equally consistent with depression and anxiety being causes or consequences of poor glycaemic control. However, structural equation modelling in a prospective study suggested that the effect of depression on symptoms of glucose dysregulation is mediated through lower adherence to self-care.100 Depression in diabetes is consistently shown to be associated with diabetes complications, including retinopathy, nephropathy, macrovascular complications, and sexual dysfunction.101 Major depression (hazard ratio [HR] 2∙3) and minor depression (HR 1∙7) are significantly associated with mortality in type 2 diabetes.102 These associations were partly but not completely explained by extensive control for behavioural mediators and diabetes severity.
Evidence for the benefits of mental health interventions on these outcomes is mixed. Meta-analyses suggest that
psychological interventions in type 1 diabetes (in children only)103 and type 2 diabetes104 improve diabetic control. Participants in these trials were generally selected on the basis of risk factors for diabetes complications, such as poor glycaemic control, obesity, or inactivity, rather than depression. A large trial in nine US primary-care clinics reported that evidence-based collaborative depression treatment (consisting of pharmacotherapy, problem-
Panel 2: Possible mechanisms for interactions between mental disorders and other health conditions
Mental disorders affect the rate of other health conditions
- Mental disorders are associated with risk factors for chronic disease such as smoking, reduced activity, poor diet, obesity, and hypertension; however, these lifestyle factors have not yet been shown to mediate associations with morbidity and mortality
- Depression has various biological effects: on serotonin metabolism117 (alteration of cardiac function, platelet aggregation, and vasoconstriction); on cortisol metabolism118 (increased cortisol, leading to inflammation, excessive clotting, and the metabolic syndrome); on inflammatory processes117,119 (raised inflammatory markers, which also predict the development of cardiovascular disease); and on cell-mediated immunity119 (impairments in T-cell mediated functions, reduced natural-killer cell counts and cytotoxicity, with relevance to cancer, HIV progression, and other infectious diseases)
- Mental disorders and other health conditions could have common genetic or environmental risk factors
Some health conditions affect the risk of mental disorders
- Some disease processes directly affect the brain. Examples include infections (eg, cerebral malaria, HIV, tuberculosis); cerebrovascular diseases (cortical strokes and progressive subcortical damage); diabetes; alcohol and substance use; and neurodevelopmental disorders. The consequences of such effects depend on the site and extent of brain damage, and can include cognitive impairment, behaviour disturbance, mood disorders, delusions, and hallucinations
- Many chronic diseases create a psychological burden, which arises from factors such as the acute trauma of the diagnosis; the difficulty of living with the illness; the long- term threat of decline and shortened life expectancy; necessary lifestyle changes; complicated therapeutic regimens; aversive symptoms such as pain; and stigma, which can lead to guilt, loss of social support, or breakdown of key relationships
- Disability associated with chronic health conditions might mediate risk for depression and other common mental disorders
Some comorbid mental disorders affect treatment and outcome for other health conditions
- Mental disorders can delay help-seeking, reduce the likelihood of detection and diagnosis, or do both
- The extent and the quality of general medical health care received by people with mental disorders might be poor.30,97,120,121 The evidence for this inequity is especially strong for those with psychoses, dementia, and substance-use disorders
- Mental disorders, cognitive impairment, and substance-use and alcohol-use disorders adversely affect adherence to medication, to recommendations for behavioural modification, and to activities to prevent disease or promote health.122 Cognitive models of illness view patients as active problem-solvers, who process health advice and make decisions that in turn influence coping, adjustment, and illness behaviour across a range of chronic illnesses.123 Cognitive behaviour therapy targets the intentions, beliefs, and attitudes of patients, and can help to modify their emotional responses and health behaviours
solving treatment, or both in combination) for patients with diabetes and depression did not produce better effects than usual primary care on either glycaemic control105 or diabetic self-care,106 despite significant effects on depression outcomes.105 Similar findings were reported from two small randomised controlled trials of antidepressant treatment in diabetes.107,108
Communicable diseases
Communicable diseases continue to cause substantial death and disability in low-income and middle-income countries. HIV/AIDS (which causes 8∙2% of all years-of- life lost) and malaria (which causes 4∙5% of years-of-life lost) collectively account for nearly 13% of premature mortality and 39% of that attributable to communicable diseases. In 2004, about 34 million people were living with AIDS and over 3 million died of the disease. Plasmodium falciparum infects 500 million people each year and causes 2∙7 million deaths, more than 90% of which are in young African children. The HIV epidemic and the emergence of strains with multiple drug-resistance has led to a resurgence of tuberculosis as a major public-health menace worldwide. In 2003, an estimated 8∙8 million new cases of tuberculosis resulted in 1∙7 million deaths; 27% of these cases and 31% of these deaths arose in Africa.109
HIV/AIDS
Some (mainly indirect) evidence shows that people with mental disorder are at heightened risk of contracting HIV/AIDS. Consistent evidence from the USA suggests that those with serious chronic mental illnesses have a high seroprevalence of HIV (5–7%), and that in those with schizophrenia, the mental illness generally precedes HIV infection.110 Behavioural risk factors identified (with a frequency of 30–60% in these high-risk groups) included high rates of sexual contact with multiple partners, low adherence to condom use, injected drug-use or sexual contact with injecting drug users, and unprotected sex between men.111 A large US cohort study of men who have sex with men provided more direct evidence: it identified use of alcohol and drugs before sex and depressive symptoms as independent predictors of seroconversion.112 Up to 10% of HIV cases worldwide are attributable to use of injection drugs.113 The evidence from low-income and middle-income countries is less clear; seroprevalence in psychiatric inpatients is often similar to that in the general population.114 Psychiatric inpatients in an Indian institution reported high rates of sexual and drug-related risk behaviours.115,116
A fairly consistent association between infection with HIV and poor mental health has been reported. Several mechanisms might be implicated. Apart from psychological trauma (panel 2)117–123 the infection itself has direct effects on the central nervous system, and causes neuropsychiatric complications;124 depression,
mania, cognitive disorder, and frank dementia, often in combination. Although the incidence of HIV-associated dementia has halved since the advent of highly active antiretroviral therapy (HAART),125 and opportunistic infections of the central nervous system are rare,125,126 the incidence of HIV encephalopathy might have risen,126 suggesting continued infiltration of the central nervous system. Evidence for neurocognitive impairment in asymptomatic HIV-infected individuals has been found,127 although the severity and number of domains affected is greater in those with symptomatic disease.128 HAART, especially with efivarenz, can be associated with a range of side-effects on the central nervous system, including depression, nervousness, euphoria, hallucination, and psychosis.110 Patients with a previous history of psychiatric disorders could be at greater risk. Death by suicide has occasionally been reported. In a national probability sample of HIV-positive men and women in the USA, the 1-year prevalence of major depression was 36% and that of generalised anxiety disorder was 16%. These prevalences are five and eight times higher, respectively, than those identified by a national household survey that used the same assessment method.129 In a meta-analysis of studies that compared HIV-positive and HIV-negative control groups130 the difference in the prevalence of major depression (9∙4% in HIV-positive vs 5∙2% in HIV-negative) was significant (OR 2∙0, 95% CI 1∙3–3∙0). A systematic review of the evidence from low-income and middle-income countries identified 13 studies of mental disorders in HIV-positive people; reported prevalence varied widely.114 The largest and best designed of these studies (which compared HIV-positive people who accessed HIV services with matched controls in Bangkok, Kinshasa, Nairobi, and Sao Paulo) reported that the rates of depressive disorder and depression symptoms were higher in symptomatic HIV-positive people, compared with either non-symptomatic cases or seronegative controls.131
Little evidence on associations between mental disorder and either help-seeking behaviour or uptake of diagnostic and treatment services for HIV/AIDS is available. In US women who were medically eligible, non-receipt of HAART was associated with substance use and with a history of childhood sexual abuse.132 Injection-drug use has consistently been shown to be associated with low uptake of HAART.113 Depression symptoms predicted drop out from a HIV-risk-reduction programme for socially deprived Latino women.133
Comorbidity affects prognosis. In US cohorts of HIV-positive women, chronic depressive symptoms were associated with increased AIDS-related mortality134,135 and with rapid disease progression,134 independent of receipt of treatment, and comorbid substance use. Impairment in cell-mediated immunity (consisting of higher activated CD8 T lymphocyte counts and lower natural killer cell activity) might be implicated (panel 2).136
Cognitive impairment in HIV has been associated with greatly increased mortality independent of baseline clinical stage, CD4 cell count, serum haemoglobin, antiretroviral treatment, and social and demographic characteristics.137 Schizophrenia complicates treatment and has been associated with poor prognosis.110 The incidence of AIDS-defining illnesses in patients on HAART was reported to be especially high in injection- drug users.113
Adherence to HAART must be almost perfect to achieve lasting viral suppression. Adherence of less than 95% independently predicts viral resistance, hospital admissions, and opportunistic infections.138 Drug resistance can be transmitted to other people, which limits treatment options. Strong and consistent evidence from treatment programmes in developed countries now shows that adherence to HAART is adversely affected by depression,138–140 cognitive impairment,141,142 and alcohol-use and substance-use disorders.113 By contrast, adherence in the presence of serious mental illnesses can be good, presumably because of close medical supervision.110 We need to know more about adherence in low-income and middle-income countries.114 One study, from Uganda, which used a diagnostic assessment for depression, reported no association with adherence,143 whereas in Ethiopia depression was associated with less than 95% self-reported adherence in the week before interview.144 Data from a non-randomised US observational cohort study showed that antiretroviral adherence improved more in 6 months for those with depression who adhered to antidepressant treatment, compared with those not treated.145 We did not find any trials of the effect of antidepressants on adherence. Randomised controlled trials of motivational interviewing (for patients with alcohol problems) and adherence interventions (for those on methadone maintenance) suggested no sustained benefit for either approach.113 Modified directly observed treatment has been shown to improve adherence by substance users in one randomised controlled trial and one controlled trial.113
Findings on the effect of psychological interventions on psychopathology and HIV prognosis have been mixed. Group cognitive behavioural interventions have been tested extensively and shown to decrease depression-symptom scores,146 reduce herpes virus IgG titres,147 improve quality of life related to mental health,148 and reduce unsafe sexual behaviours.149 The evidence base for antidepressant treatment is surprisingly small, with only a few small randomised controlled trials and a much larger number of open-label interventions.124 Both tricyclic antidepressants and SSRI antidepressants seem to improve depression symptoms but have no effect on CD4 cell counts.150–152 Coverage and uptake are a challenge even in the USA, which has substantial resources; in a national survey of HIV-positive care recipients, about half those with depressive disorders did not receive antidepressants.153
Very few studies have investigated mental disorder as a predictor for HIV transmission, especially since the advent of HAART. One study of 168 HIV-infected men with resistance to antiretroviral drugs showed a high rate of high-risk sexual behaviour (such as unprotected anal or vaginal intercourse with an HIV-uninfected or status-unknown partner).154 These investigators reported strong evidence that depression, younger age, and sildenafil use predicted transmission, and moderate evidence that frequent alcohol use did so.
Tuberculosis
People with mental and substance use disorders might be at increased risk of contracting tuberculosis, although few studies have investigated this topic. A case registry study from Nagasaki suggested that the incidence of tuberculosis in patients with schizophrenia was high;155 similarly, high infection rates were recorded in people with serious mental illness in a psychiatric day programme in New York.156 Occasional reports of outbreaks in inpatients suggest that institutionalisation might contribute to risk of tuberculosis.157 A US population-based case-control study reported that heavy drinkers had twice the risk of tuberculosis infection of non-drinkers.158 Poor adherence to antituberculosis medication is an important barrier to global control of the disease, and increases the risks of morbidity, mortality, and drug resistance in both individuals and communities.159 Since treatment for multidrug-resistant tuberculosis is long (generally 2 years) and painful (consisting of daily injections for at least 6 months), with many unpleasant side-effects, adherence can be a challenge. A review of 13 treatment cohort analyses identified treatment-default rates of up to 39%, with an average of 12∙6%.160 Alcohol-use disorder has also been reported to be associated with delayed treatment-seeking in Kiev; with poor adherence to directly observed therapy in New York;161,162 with unfavourable treatment outcomes for pulmonary tuberculosis in Kazakhstan163 and for multidrug-resistant tuberculosis in Tomsk, Russia;156 and with increased mortality in a US trial of directly observed therapy (HR 2∙9).164 We identified only one report that did not find an association between psychiatric illness and substance use and poor adherence to tuberculosis treatment in a sample of homeless adults.165
Since depression has an important effect on adherence to treatment for many health conditions, the amount of research into comorbidity between tuberculosis and common mental disorders is surprisingly low. Multidrug-resistant tuberculosis, in particular, might be associated with poor mental health, attributed variously to loss of work and social roles and feelings of hopelessness and stigma.166 In Peru, the incidence of depressive disorder at recruitment into a treatment programme for multidrug-resistant tuberculosis was 52%, with further incidences of 13∙3%, 12∙0%, and 12∙0% for depression, anxiety, and psychosis, respectively,
during treatment.167 In an inpatient study in Turkey, the prevalence of depression, anxiety, or both was assessed to be 19% for recently diagnosed tuberculosis, 22% for defaulted tuberculosis, and 26% for multidrug-resistant tuberculosis.168 The prevalence of common mental disorders in 53 Nigerian tuberculosis patients recruited in a chest clinic was 30%, compared with 5% in healthy controls.169 A community-based study in Mali had suggested an even stronger association (OR 9∙3), but with self-reported tuberculosis episodes.170
The failure of directly observed therapy to deliver improvement in treatment completion or cure171 has led to calls for rigorous investigation of extended interventions that address other factors known to influence adherence, such as quality of communication with treatment providers, patients’ health beliefs, patients’ education, and economic barriers.122 Since patients with multidrug-resistant tuberculosis face a range of difficulties, the development of strategies to support these patients will be essential to ensure treatment adherence. The information–motivation–behavioural skills model, which was originally developed to modify HIV-risk behaviour, has been recommended for use in tuberculosis treatment.122 Interventions based on cognitive behaviour therapy, which have proved helpful in management of various chronic diseases, have many similarities. In Peru, a non-randomised assessment of a group psychotherapy intervention, coupled with recreation, symbolic celebrations, and family workshops, was associated with a default rate of only 3∙5% in a treatment cohort of 276 patients with multidrug-resistant tuberculosis.172 In India, a psychotherapeutic intervention based on behavioural-modification techniques was tested in a blind controlled trial with alternate allocation.173 Those who participated in the intervention were more likely than controls to complete treatment (72% vs 42%) and to be cured (72% vs 42%), and were less likely to default (17% vs 53%).173 The cost of the intervention was US$20 per patient, which was a quarter of the cost of the medication. In another non-randomised controlled trial, in Ethiopia, patients in tuberculosis clubs had significant improvements in treatment completion (69% vs 47%) and lower default rates (13% vs 41%), compared with controls.174
Malaria
No studies have investigated the possibility that mental disorders might increase susceptibility for malaria. Possible mechanisms could include effects on immunity, and on adherence to effective preventive measures. Severe falciparum malaria is associated with self-limiting psychiatric disorders,175 including depression,176,177 schizophrenic and manic syndromes, anxiety attacks,178 and confusional episodes.179 Treatment, especially with chloroquine, might be an associated factor.180 These syndromes might complicate and delay diagnosis.171 The extent of comorbidity between mental disorders and
recurrent episodes of malaria, parasitaemia, or both has been very little studied. Dugbartey and colleagues181 compared 142 adult Ghanaians who had had a documented episode of malaria at least 12 months before the study with 30 community controls who had full medical records, no history of record of infection, and no existing parasitaemia. Patients with malaria had high scores for anxiety, depression, and total psychological symptoms, compared with controls.181 Carta and colleagues,170 in a small cross-sectional community survey in Mali, reported no association between acute malaria and common mental disorder. A systematic review provided strong evidence that malaria has both short-term effects on cognitive function and longer-term effects on cognitive development in children.182 Impairment is associated with the severity of the infection; cerebral malaria is especially important. Effects of non-severe malarial disease might be mediated through disrupted school attendance.182 In adults, a 1-year follow-up of cerebral malaria cases in Ghana reported no deficits,181 and a 20-year follow-up of Vietnam war veterans reported deficits in memory, language, and attention.183
By comparison with work on tuberculosis, research on the effect of mental health on the prevention and effective treatment of malaria is scarce. Antimalaria programmes focus on intensification of preventive measures,184 (including use of insecticide-treated nets, which can reduce episodes of malaria in children by 50%),185 and encouragement of access to and uptake of affordable treatment within 24 h of onset.184 Recognised barriers to adoption of preventive health measures include poverty, inadequate education,186–188 knowledge and beliefs about malaria, and the complexity of preventive measures.188 For children, women are often the first to recognise the illness and have responsibility for illness management, although they might not have decisionmaking or financial control. Although the effects of illness-beliefs and attributions on help-seeking189 and self-treatment are increasingly well understood,190 three reviews189–191 suggest that mental health has not been regarded as relevant to help-seeking or self-treatment. Patient adherence is a major determinant of the therapeutic response to antimalarial drugs. A systematic review of 24 studies192 concluded that adherence was improved by interventions which focused on provider knowledge and behaviour, packaging, and provision of correct dosages. None of these studies discussed whether patients’ mental health (or maternal mental health status for children) would affect adherence to treatment.
Inappropriate overdiagnosis of malaria is also well documented: adverse consequences include drug side-effects, drug resistance, increased health-care costs, and failure to treat other causes of fever.193 In Africa, more than 70% of patients with suspected cases of malaria diagnose and manage their illness with traditional remedies or non-prescription drugs.193 A review suggests an average overestimation of 61% (range 32–96%) for
clinical diagnosis, compared with a microscopy-based gold-standard diagnosis.193 In one series, 40% of those given a clinical diagnosis did not present with pyrexia.194 Somatisation might well account for a proportion of misdiagnosed cases.
Reproductive and sexual health
Women are at heightened risk for common mental disorders: a female to male sex ratio of 1∙5 to 2∙0 is typical.195,196 In Pakistan, the prevalence of common mental disorders in men is similar to that in other regions, but women are two to three times more likely than men to suffer from such disorders.197 Gender affects many of the determinants of mental health, including socioeconomic position, access to resources, social roles, rank, and status; and gender differences in mental disorders diminish after controlling for these mediators.195,198 The gendered disadvantage experienced by women in many parts of the world199 might be a relevant factor; for example, a large cross-sectional survey in Goa, India identified strong associations between common mental disorders and indicators of disadvantage, including early age at marriage, intimate partner violence and abuse, and absence of decisionmaking autonomy.200
A systematic review identified 122 studies of the association between mental disorder and gynaecological morbidity.201 Sexual and other forms of abuse, anxiety, depression, and use of substances and alcohol were robustly and consistently reported to be associated with various reproductive health outcomes, including dysmenorrhoea, dyspareunia, and non-cyclical pelvic pain.200 Studies in south Asia, where abnormal vaginal discharge is a common complaint, report similar associations.200,202 Gynaecological complaints might be somatic idioms for common mental disorders; in the Goa study, the complaint of vaginal discharge was associated with symptoms of common mental disorder (OR 2∙2, 95% CI 1∙4–3∙2) and somatoform disorders (6∙2, 4∙0–9∙7), but not with reproductive-tract infection diagnosed with gold-standard laboratory tests (1∙2, 0∙9–1∙6).203 In Asian cultures, explanatory models of reproductive health and mental health experiences might enhance the association between these health domains.
Maternal and child health
Maternal psychosis affects infant growth and survival. Maternal schizophrenia is consistently associated with preterm delivery204,205 and low birthweight.204–206 The effect of maternal psychosis on child survival has also been investigated—a meta-analysis linked maternal psychosis with a two-fold increased risk of stillbirth or infant mortality.207 Postpartum depression affects 10% to 15% of women in developed countries,208 with adverse consequences for the early mother–infant relationship and for children’s psychological development.209 In low-income and middle-income countries, the prevalence
of perinatal depression is, if anything, somewhat higher than in the developed world.210,211 Physical development of infants is a particular problem in Asia.212 An independent association between antenatal common mental disorder and low birthweight has been shown by two prospective studies: one from Pakistan (RR 1∙9, 95% CI 1∙3–2∙9)213 and one from India (OR 1∙4, 95% CI 1∙0–2∙1).214 Findings from high-income countries have been equivocal, with several negative reports.215 However, associations between maternal depression and preterm birth216 and between psychosocial stressors and low birthweight,217 were reported from a disadvantaged African–American community.
In south Asia, two case-control and two cohort studies have consistently shown associations between perinatal common mental disorders and infant undernutrition at
6 months, after controlling for birthweight.211,218–221 However these studies did not assess the relative, independent contributions of antenatal and postnatal common mental disorders, and only one controlled for maternal nutrition.219 In the cohort study from Pakistan, 6-month old infants of antenatally depressed mothers were at much higher risk of being underweight (RR 4∙0, 95% CI 2∙1–7∙7) and stunted (4∙4, 1∙7–11∙4), after adjusting for birthweight, socioeconomic status, and frequent diarrhoea.219 In the same study, children of antenatally depressed mothers were also more likely to have had more than five diarrhoeal episodes in the first year of life (RR 2∙3, 95% CI 1∙6–3∙1).222 In South Africa, neither postnatal nor current depression was associated
with infant growth at 2 months, after adjusting for birthweight; however, there was a non-significant association at 18 months, and the study was small and had low power.223 A multicountry study that assessed commonmentaldisordersinmotherscontemporaneously with child growth at 6–18 months postpartum reported no cross-sectional association, in Ethiopia, between maternal mental health and child malnutrition, but did note that common mental disorders in mothers were associated with infant stunting in India, and with underweight infants in Vietnam.224 The longer-term effects of maternal mental health on infant growth or mortality have not yet been studied in low-income and middle-income countries.
A Lancet review reported that the effect of maternal depression on child cognitive development has been studied less extensively in low-income and middle-income countries than in developed countries.225 In south India, maternal postnatal common mental disorder was negatively associated with mental-development quotient scores in infants at 6 months, but not with motor development.211 In Barbados, a long-term prospective study reported associations between maternal common mental disorder and impaired cognitive and motor development in infants at 6 months,226 and poor performance in high-school entrance examinations in children aged 11–13 years.227
Strong but not consistent evidence from developed countries shows that maternal depression reduces adherence to child-health promotion and prevention
|
|
|
MD is a risk factor for the HC |
MD is a consequence of the HC |
Comorbidity (uncertain causal direction) |
MD affects adherence to treatment for HC |
MD affects prognosis or outcome of the HC |
Treatment for MD affects mental health in those with HC |
Treatment for MD affects physical HC |
|
|
Non-communicable diseases |
|
|
|
|
|
|
|
|
|
|
Depression and CMD with coronary heart disease |
4 |
3 |
3 |
2 |
3 |
1 |
−1 |
|
|
|
Depression with stroke |
3 |
3 |
3 |
0 |
3 |
−1 |
−1 |
|
|
|
Common mental disorder with diabetes |
1 |
2 |
3 |
3 |
3 |
1 |
1 |
|
|
|
Schizophrenia with diabetes |
1 |
1 |
3 |
2 |
0 |
0 |
0 |
|
|
|
Communicable diseases |
|
|
|
|
|
|
|
|
|
|
Depression and CMD with HIV/ AIDS |
2 |
2 |
4 |
3 |
3 |
3 |
1 |
|
|
|
Serious mental illness with HIV/AIDS |
1 |
3 |
3 |
1 |
2 |
0 |
0 |
|
|
|
Cognitive impairment and dementia with HIV/AIDS |
0 |
3 |
3 |
3 |
2 |
0 |
0 |
|
|
|
Alcohol-use and substance use disorder with HIV/AIDS |
2 |
0 |
3 |
3 |
3 |
0 |
2* |
|
|
|
CMD with malaria |
0 |
2 |
2 |
0 |
0 |
0 |
0 |
|
|
|
Cognitive impairment with malaria |
0 |
4 |
NA |
0 |
0 |
0 |
0 |
|
|
|
Alcohol-use disorder with tuberculosis |
2 |
0 |
2 |
3 |
3 |
0 |
0 |
|
|
|
Depression or common mental disorder with tuberculosis |
0 |
2 |
2 |
3 |
0 |
0 |
2 |
|
|
|
Maternal and child health |
|
|
|
|
|
|
|
|
|
|
Maternal depression and CMD with impaired child growth and development |
3 |
0 |
1 |
0 |
0 |
NA |
0 |
|
|
|
|
Maternal psychosis with infant mortality |
4 |
0 |
NA |
NA |
NA |
NA |
0 |
|
measures, including up-to-date vaccination,228,229 receipt of well-child visits,229 and use of the recommended back-sleeping position.230,231 In Pakistan, maternal antenatal depression was associated with failure to update infant immunisation at 1 year.219 Good evidence from both developed countries230 and low-income and middle-income countries232 shows that maternal depression is associated with suboptimal breastfeeding. We need more evidence about effects on children’s receipt of health care. However, in the US, a large national cohort study229 showed that maternal depression was independently associated with increased hospital admissions and emergency department visits in children (OR 1∙4, 95% CI 1∙2–1∙8), in line with findings from smaller studies.230,233 This evidence suggests that help-seeking for potentially serious childhood illnesses might be delayed when mothers are depressed.
Injuries
Injury and violence are important causes of death and disability worldwide. The 2005 WHO report estimated that 5∙4 million deaths from injury accounted for 9% of deaths worldwide and 12% of the global burden of disease, and that such deaths would increase substantially by 2030.4 Mental health problems are both a cause and a consequence of injury. Injury and mental disorder also have many determinants in common, such as poverty,234,235 conflict, violence, and alcohol use. Any public-health approach to injury control must consider mental health. Road-traffic accidents are responsible for about 1∙2 million deaths and perhaps ten times as many permanent disabilities each year.236 Three-quarters or more of the deaths are in developing countries, where numbers of accidents and fatalities have been increasing at an alarming rate.237 Within low-income and middle-income countries, poor people (pedestrians, passengers in buses and trucks, and cyclists) suffer a higher burden of morbidity and mortality from traffic injuries.238 In 1964, a US study showed that alcohol was a strong risk factor for involvement in road traffic accidents,239 and this finding has been substantiated by subsequent epidemiological studies. Although data are scarce, alcohol is implicated in a large proportion of road traffic accident deaths in low-income and middle-income countries.237 Nevertheless, variations between countries are apparent; in China the proportion of alcohol-related traffic accidents might be as low as 1%.240 A proportion of unintentional injuries might be unrecognised suicide attempts; a US study noted that the rate of suicide was ten times higher in those with at least one previous hospital admission for injury, and almost three times higher for drivers who had been injured in a road traffic
accident.241
Earlier reports of cross-sectional associations between maternal depression and child injury risk242,243 have been supported by the findings of a 10 000-family cohort study in the UK;244 maternal postnatal depression was
prospectively and independently associated with burns or scalds (1∙29, 95% CI 1∙01–1∙64) and with two or more accidents during the follow-up period (1∙39, 1∙16–1∙66).244 Up to 98% of child injury deaths happen in low-income and middle-income countries;3 one study reported strong and consistent cross-sectional associations between common mental disorder in caregivers and injuries in children in India, Peru, Vietnam, and Ethiopia.245 Evidence for an inverse association between maternal depression and self-reported accident-prevention practices is less consistent. US studies reported an inverse association with preventive practices (such as use of car seatbelts246,247 and electrical plug covers246), but a UK study found no association in socioeconomically deprived families with practices such as use of fireguards, stair gates, smoke alarms, window locks, or safe storage of medicines.248
Injury and violence are also important risk factors for mental disorder. Post-traumatic stress disorder is a recognised consequence of non-intentional injury; analysis of data from the 1958 British birth-cohort study showed that injury and burns were strongly associated with psychological distress.249,250 Child abuse is a potent risk factor for psychiatric disorders and suicidal behaviour; intimate partner violence is a risk factor for depression, anxiety, and suicide; sexual violence is a risk factor for mental health and behavioural problems; and collective violence is a risk factor for depression, substance abuse, and suicide.251,252 Conflict was responsible for an estimated 184 000 deaths in 2005.3 Post-traumatic stress disorder is a common psychological outcome of conflict, with a quarter or more of survivors affected;253
(Continued from previous page)
To model prevention of infant stunting in Pakistan we based our calculations on a 25% prevalence of depression in mothers and a relative risk of 4·4 for the association of maternal depression with infant stunting at 6 months.219 We predicted that up to 8% of stunting would be averted at 25% coverage, rising to 20% at 75% coverage. Since 92 000 stunted infants are born each year in Pakistan (comprising 31% of all births), this estimate would translate into a maximum of 13 800 cases of stunting averted each year, if 50% coverage could be achieved (figure 2). The developmental and health consequences of stunting are expected to decrease an adult’s yearly income by 20% (US$144 at current income per head) which would imply a nationwide saving of $US1·99 million every year. Furthermore, for a 10% reduction in the number of stunted children, the number of children who completed primary school education would be expected to increase by 8%.266
Figure 2: Proportion of health problems theoretically prevented by increased coverage of evidence-based treatment for depression
To model prevention of suicide in China we based our calculations on a 4·3% prevalence of major depression267 and a relative risk of 14·6 for the association of depression with suicide.23 We predicted that a maximum of 6% of suicides would be averted at
25% coverage of the intervention, rising to 15% at 75% coverage (figure 2). Since
325 581 suicides happen every year in China, we estimated that if 50% coverage with the intervention could be achieved, a maximum of 32 558 suicides would be averted every year. The potential economic effect could be substantial, with 5·8 million productive life- years lost nationally, which would translate to lost productivity of US$10·2 billion per year because of suicide (on the basis of GDP per head of US$1740 in 2006). If 50% treatment coverage was achieved, a 10% reduction in the suicide rate would save
580 000 productive years of life, or US$1·0 billion per year. Alternatively, we used willingness-to-pay estimates268 and the estimate of US$34 458 as the value of a statistical life in China, to calculate a saving of US$1·1 billion per year.
prevalence of post-traumatic stress disorder rises with the number of traumatic events witnessed.254,255 These effects are still discernable in displaced refugees up to 20 years after a conflict.256,257 Apart from post-traumatic stress disorder, common mental disorders are also very frequently reported in populations after conflicts and complex emergencies,253 even in those who have not been directly exposed to violence.253
Implications for policy, practice, and research WHO estimates of the global burden of disease have helped to raise awareness of the enormous effect of mental disorders, both in their own right and relative to other
health conditions. Much of this effect arises from the commonest disorders, especially depression and alcohol-use disorder. However, the Cartesian dualism that is implicit in the methods used to generate these estimates has meant that what began as a blessing is now, in some respects, a bane. In reality, the interactions between mental disorders and other health conditions are widespread and complex (table 2). Mental disorders are risk factors for the development of communicable and non-communicable diseases, and contribute to accidental and non-accidental injuries. For some infectious diseases, mental disorders in infected persons increase the risk for transmission. Many health conditions increase the risk for mental disorder, or lengthen episodes of mental illness. The resulting comorbidity complicates help-seeking, diagnosis, quality of care provided, treatment, and adherence, and affects the outcomes of treatment for physical conditions, including disease-related mortality. For many health conditions, mental illness makes an independent contribution to disability and quality of life.
Mental health is missing from the policy framework for health improvement –and poverty reduction; missing from health and social research; and missing from targets for interventions. Moreover, mental health has not been acknowledged as an obstacle to achievement of several Millennium Development Goals—notably, promotion of gender equality and empowerment of women, reduction of child mortality, improvement of maternal health, and reversal of the spread of HIV/AIDS, malaria, and other diseases.
Mental health awareness needs to be integrated into all elements of health and social policy, health-system planning, and health-care delivery. Sophisticated evidence- based arguments to increase resources for mental health care should be linked to evidence for its wider importance to public health.258 Integrated mental health policies, applied across disease categories, and to different levels of care and types of care setting, will maximise the effectiveness of the small number of mental health professionals available in most low-income and middle- income countries.259 Such policies will also mobilise the forces of public and community health to work for better mental health and reduce redundancies and budgetary and organisational inefficiencies in overstretched health systems. The strengthening of health-care systems to deliver mental health care should focus, where possible, on existing programmes and activities such as HIV prevention, antiretroviral treatment programmes, treatment of multidrug-resistant tuberculosis, campaigns against gender-based violence, antenatal care, integrated management of childhood illness, and innovative chronic- disease management.260
Mental health needs to be recognised as an integral component of practice in primary and secondary health care. Beyond this, primary health-care workers need to be trained in recognition and evidence-based treatment of mental disorders, and given suitable supervision and
support. Basic drug and psychotherapeutic treatments need to be made available at all levels of health care—the evidence for treatment of specific disorders is presented later in this Series.261 Primary and secondary care providers should overcome their reluctance to treat patients with severe mental illnesses, and learn effective ways to interact and communicate with these patients. Inequities in access and provision of good-quality physical-health care for people with mental disorders must be ended. We need to promote holistic models of care, which integrate psychosocial assessments and interventions seamlessly and routinely into the management protocols for major communicable and non-communicable diseases and reproductive and childhood disorders. For example, our modelling exercises indicated that up to 20% of infant stunting could be averted if maternal depression was treated more effectively, and that up to 15% of suicides could be averted by interventions to treat major depression (panel 3). By the same token, mental health professionals should routinely assess their patients to identify and monitor physical-health problems, should encourage them to attend regular checks in primary care, and should generally place a greater emphasis on lifestyle review and management. Current guidelines about the management of patients given antipsychotic drugs should be applied; for example, patients with schizophrenia should be weighed at every visit. Although more mental health specialists are needed, these might never be sufficient to meet the need, especially in low-income countries. The marshalling of this scarce resource will demand careful thought and planning, including clear protocols for referral from primary care.
Evidence for interactions between mental health and other health conditions comes overwhelmingly from the developed world, especially the USA. Whereas 80% of deaths from non-communicable diseases are in low-income and middle-income countries, all but four of the 59 papers cited in the non-communicable disease section of this review describe research from north America and Europe. Although 99% of deaths from HIV/AIDS are in low-income and middle-income countries, nearly all research on the interaction between mental disorders and chronic management of HIV infection comes from the USA. 99% of deaths from malaria are in low-income countries and 90% of these are in children aged younger than 5 years; we identified an absence of evidence, rather than evidence of absence, for what could be, by analogy with other evidence, important interactions between maternal mental health, adherence to malaria prevention measures, and prompt and appropriate help-seeking for childhood infections.
The first priority, therefore, is to increase the evidence-base for interactions between mental health and other health conditions in low-income and middle-income countries. Some existing evidence (eg, that which investigates mental disorders as risk factors and prognostic indicators for non-communicable
diseases) might be generalisable to less well developed settings. However, the evidence on maternal depression and infant growth outcomes is reported mainly from low-income and middle-income countries. Only research that is conducted locally can be expected to affect awareness and lead to new policy development.
Second, we need to understand better the mechanisms that underlie interactions between mental health and other health conditions, if we are to develop effective public-health and clinical interventions (panel 2). We need to learn from the experience that, in many instances, interventions designed to treat common mental disorders are effective for reduction of the frequency of these conditions, but not for improvement of downstream physical-health outcomes with which associations had been reported.76–79,105–108,150–152 Explicit targeting of illness representations and associated behaviours through cognitive behavioural techniques might be effective.
Third, we are as yet at a very early stage in the development and trialling of adjunctive psychosocial, psychological, and mental health interventions. Despite strong evidence for relevance of mental health to HIV/AIDS, well designed trials to investigate effects of mental illness on the important downstream health outcomes are scarce; for example, presentation for voluntary testing and counselling, access to and acceptance into HAART programmes, adherence, adoption of low-risk behaviours, virological and immune status, and survival.
We have stressed the potential capacity for psychosocial interventions to improve physical-health outcomes (eg, as shown for glycaemic control in diabetes,104 and as modelled in panel 3 for infant stunting in Pakistan,219 and suicide prevention in China23). However, we need also to act immediately on the existing robust evidence that treatment of comorbid mental disorder is highly effective for improvement of mental health and quality of life outcomes across a range of disorders including cancer,269 diabetes,105 heart disease,76,79 and HIV/AIDS.146,148 The moral and ethical case for redressing the imbalance in provision for people with mental disorders can brook no delay.270 Practical steps such as those discussed in this Series must be accompanied, wherever possible, by high quality assessments of efficacy and cost-effectiveness.
Acknowledgments
We thank the Lancet Global Mental Health Group, Michael Dewey for advice on the modelling exercise, and Simon Wessely and Ian Roberts for comments and contributions to the draft manuscript. VP is supported by a Wellcome Trust Senior Clinical Research Fellowship in Tropical Medicine. AR is supported by a Wellcome Trust Career Development Fellowship in Tropical Medicine. The Lancet Global Mental Health Series was supported by a grant from the John and Catherine MacArthur Foundation. SS is an employee of WHO; the views expressed in this article do not necessarily represent the decisions, policy, or views of WHO.
References
- WHO. Mental health: facing the challenges, building solutions. Report from the WHO European Ministerial Conference. Copenhagen, Denmark: WHO Regional Office for Europe, 2005.
- WHO. International Statistical Classification of Diseases and Related Health Problems, 10th revision. Geneva, Switzerland: World Health Organization, 1992–94.
- Murray CJL, Lopez AD, eds. The global burden of disease and injury series, volume 1: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020.Cambridge, MA, USA: Harvard University Press, 1996.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006, 3: e442. doi: 10.1371/journal.pmed.00304424
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health 1994; 84: 1796–99.
- Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA 1998; 279: 1720–26.
- Phifer JF, Murrell SA. Etiologic factors in the onset of depressive symptoms in older adults. J Abnorm Psychol 1986; 95: 282–91.
- Kennedy GJ, Kelman HR, Thomas C. The emergence of depressive symptoms in late life: the importance of declining health and increasing disability. J Commun Health 1990; 15: 93–104.
- Beekman ATF, Deeg DJH, Smit JH, van Tilburg W. Predicting the course of depression in the older population: results from a community-based study in the Netherlands. J Affect Disord 1995; 34: 41–49.
- Prince MJ, Harwood RH, Thomas A, Mann AH. A prospective population-based cohort study of the effects of disablement and social milieu on the onset and maintenance of late-life depression. The Gospel Oak Project VII. Psychol Med 1998; 28: 337–50.
- Schoevers RA, Beekman AT, Deeg DJ, et al. Risk factors for depression in later life; results of a prospective community based study (AMSTEL). J Affect Disord 2000; 59: 127–37.
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis.
Am J Psychiatry 2003; 160: 1147–56.
- Ormel J, Kempen GI, Penninx BW, Brilman EI, Beekman AT,
van Sonderen E. Chronic medical conditions and mental health in older people: disability and psychosocial resources mediate specific mental health effects. Psychol Med 1997; 27: 1065–77.
- Broe GA, Jorm AF, Creasey H, et al. Impact of chronic systemic and neurological disorders on disability, depression and life satisfaction. Int J Geriatr Psychiatry 1998; 13: 667–73.
- Braam AW, Prince MJ, Beekman AT, et al. Physical health and depressive symptoms in older Europeans. Results from EURODEP. Br J Psychiatry 2005; 187: 35–42.
- Beekman AT, Penninx BW, Deeg DJ, Ormel J, Braam AW,
van Tilburg W. Depression and physical health in later life: results from the Longitudinal Aging Study Amsterdam (LASA).
J Affect Disord 1997; 46: 219–31.
- Carroll LJ, Cassidy JD, Cote P. Factors associated with the onset of an episode of depressive symptoms in the general population.
J Clin Epidemiol 2003; 56: 651–58.
- Bruce ML, Hoff RA. Social and physical health risk factors for first-onset major depressive disorder in a community sample. Soc Psychiatry Psychiatric Epidemiol 1994; 29: 165–71.
- Aaron R, Joseph A, Abraham S, et al. Suicides in young people in rural southern India. Lancet 2004; 363: 1117–18.
- Prasad J, Abraham VJ, Minz S, et al. Rates and factors associated with suicide in Kaniyambadi Block, Tamil Nadu, South India, 2000–2002. Int J Soc Psychiatry 2006; 52: 65–71.
- Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med 2003; 33: 395–405.
- Vijayakumar L, Rajkumar S. Are risk factors for suicide universal? A case-control study in India. Acta Psychiatr Scand 1999; 99: 407–11.
- Phillips MR, Yang G, Zhang Y, Wang L, Ji H, Zhou M. Risk factors for suicide in China: a national case-control psychological autopsy study. Lancet 2002; 360: 1728–36.
- Saz P, Dewey ME. Depression, depressive symptoms and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry 2001; 16: 622–30.
- Blazer DG, Hybels CF, Pieper CF. The association of depression and mortality in elderly persons: a case for multiple, independent pathways. J Gerontol A Biol Sci Med Sci 2001; 56: 505–09.
- Abas M, Hotopf M, Prince M. Depression and mortality in a high-risk population. 11-Year follow-up of the Medical Research
Council Elderly Hypertension Trial. Br J Psychiatry 2002; 181: 123–28.
- Heila H, Haukka J, Suvisaari J, Lonnqvist J. Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychol Med 2005; 35: 725–32.
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001; 58: 844–50.
- Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry 2001; 16: 751–61.
- Lawrence DM, Holman CD, Jablensky AV, Hobbs MS. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980–1998. Br J Psychiatry 2003; 182: 31–36.
- Mogga S, Prince M, Alem A, et al. Outcome of major depression in Ethiopia: population-based study. Br J Psychiatry 2006; 189: 241–46.
- Kebede D, Alem A, Shibre T, et al. Short-term symptomatic and functional outcomes of schizophrenia in Butajira, Ethiopia. Schizophr Res 2005; 78: 171–85.
- White IR, Altmann DR, Nanchahal K. Mortality in England and Wales attributable to any drinking, drinking above sensible limits and drinking above lowest-risk level. Addiction 2004; 99: 749–56.
- Shkolnikov V, McKee M, Leon DA. Changes in life expectancy in Russia in the mid-1990s. Lancet 2001; 357: 917–21.
- Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993; 153: 2474–80.
- Kroenke K, Spitzer RL, Williams JB, et al. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med 1994; 3: 774–79.
- Russo J, Katon W, Sullivan M, Clark M, Buchwald D. Severity of somatization and its relationship to psychiatric disorders and personality. Psychosomatics 1994; 35: 546–56.
- Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med 2003; 65: 528–33.
- Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005; 62: 903–10.
- Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: a study in primary care. Psychosom Med 1998; 60: 466–72.
- Gureje O, Simon GE, Ustun TB, Goldberg DP. Somatization in cross-cultural perspective: a World Health Organization study in primary care. Am J Psychiatry 1997; 154: 989–95.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64: 258–66.
- Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry 1995; 52: 238–43.
- Dickinson WP, Dickinson LM, deGruy FV, Main DS, Candib LM, Rost K. A randomized clinical trial of a care recommendation letter intervention for somatization in primary care. Ann Fam Med 2003; 1: 228–35.
- O’Malley PG, Jackson JL, Santoro J, Tomkins G, Balden E, Kroenke K. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract 1999; 48: 980–90.
- Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychother Psychosom 2000; 69: 205–15.
- Allen LA, Woolfolk RL, Escobar JI, Gara MA, Hamer RM. Cognitive-behavioral therapy for somatization disorder: a randomized controlled trial. Arch Intern Med 2006; 166: 1512–18.
- Sumathipala A, Hewege S, Hanwella R, Mann AH. Randomized controlled trial of cognitive behaviour therapy for repeated consultations for medically unexplained complaints: a feasibility study in Sri Lanka. Psychol Med 2000; 30: 747–57.
- Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet 2005; 366: 1578–82.
- Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ 1999; 318: 1460–67.
- Kuper H, Marmot M, Hemingway H. Systematic review of prospective cohort studies of psychosocial factors in the etiology and prognosis of coronary heart disease. Semin Vasc Med 2002; 2: 267–314.
- Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000; 62: 463–71.
- Larson SL, Owens PL, Ford D, Eaton W. Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke 2001; 32: 1979–83.
- Ohira T, Iso H, Satoh S, et al. Prospective study of depressive symptoms and risk of stroke among Japanese. Stroke 2001; 32: 903–08.
- Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998; 158: 1133–38.
- Simon GE, Von KM, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population.
Arch Gen Psychiatry 2006; 63: 824–30.
- Degenhardt L, Hall W. The relationship between tobacco use, substance-use disorders and mental health: results from the National Survey of Mental Health and Well-being. Nicotine Tob Res 2001; 3: 225–34.
- Kandel DB, Huang FY, Davies M. Comorbidity between patterns of substance use dependence and psychiatric syndromes.
Drug Alcohol Depend 2001; 64: 233–41.
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity—a population-based study including smoking cessation after three years. Drug Alcohol Depend 2004; 76: 287–95.
- de LJ, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76: 135–57.
- Lewinsohn PM, Rohde P, Seeley JR, Fischer SA. Age and depression: unique and shared effects. Psychol Aging 1991; 6 (2): 247–60.
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry 2004; 55: 69–76.
- Patel V, Kirkwood BR, Pednekar S, Weiss H, Mabey D. Risk factors for common mental disorders in women. Population-based longitudinal study. Br J Psychiatry 2006; 189: 547–55.
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA 1990; 264: 1541–45.
- Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med 1997; 6: 43–49.
- Strik JJ, Lousberg R, Cheriex EC, Honig A. One year cumulative incidence of depression following myocardial infarction and impact on cardiac outcome. J Psychosom Res 2004; 56: 59–66.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160: 1818–23.
- Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction.
J Am Coll Cardiol 2003; 42: 1801–07.
- Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry 2003; 60: 627–36.
- Aben I, Lodder J, Honig A, Lousberg R, Boreas A, Verhey F. Focal or generalized vascular brain damage and vulnerability to depression after stroke: a 1-year prospective follow-up study.
Int Psychogeriatr 2006; 18: 19–35.
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Vascular risk factors and incident late-life depression in a Korean population. Br J Psychiatry 2006; 189: 26–30.
- Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study.
J Am Geriatr Soc 2004; 52: 774–78.
- Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol 1990; 47: 785–89.
- Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke 2001; 32: 113–17.
- Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality.
Am J Psychiatry 1993; 150: 124–29.
- Rees K, Bennett P, West R, Davey SG, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev 2004; 2: CD002902.
- Strik JJ, Honig A, Lousberg R, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a
double-blind, placebo-controlled trial. Psychosom Med 2000; 62:
783–89.
- Glassman AH, O‘Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288: 701–09.
- Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289: 3106–16.
- Frasure-Smith N, Lesperance F. Depression—a cardiac risk factor in search of a treatment. JAMA 2003; 289: 3171–73.
- Anderson CS, Hackett ML, House AO. Interventions for preventing depression after stroke. Cochrane Database Syst Rev 2004; 2: CD003689.
- Hackett ML, Anderson CS, House AO. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke 2005; 36: 1098–103.
- Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry 2003; 160: 1823–29.
- Golden SH, Williams JE, Ford DE, et al. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2004; 27: 429–35.
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care 1996; 19: 1097–102.
- Saydah SH, Brancati FL, Golden SH, Fradkin J, Harris MI. Depressive symptoms and the risk of type 2 diabetes mellitus in a US sample. Diabetes Metab Res Rev 2003; 19: 202–08.
- Holt RI, Bushe C, Citrome L. Diabetes and schizophrenia 2005: are we any closer to understanding the link? J Psychopharmacol 2005; 19: 56–65.
- Cohn TA, Remington G, Zipursky RB, Azad A, Connolly P, Wolever TM. Insulin resistance and adiponectin levels in drug-free patients with schizophrenia: a preliminary report. Can J Psychiatry 2006; 51: 382–86.
- Cohen D, Stolk RP, Grobbee DE, Gispen-de Wied CC. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care 2006; 29: 786–91.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24: 1069–78.
- Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res 2002; 53: 1053–60.
- Dolder CR, Lacro JP, Jeste DV. Adherence to antipsychotic and nonpsychiatric medications in middle-aged and older patients with psychotic disorders. Psychosom Med 2003; 65: 156–62.
- Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000; 160: 3278–85.
- Lin EH, Katon W, Von KM, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004; 27: 2154–60.
- Johnson KH, Bazargan M, Bing EG. Alcohol consumption and compliance among inner-city minority patients with type 2 diabetes mellitus. Arch Fam Med 2000; 9: 964–70.
- Jones LE, Clarke W, Carney CP. Receipt of diabetes services by insured adults with and without claims for mental disorders. Med Care 2004; 42: 1167–75.
- Desai MM, Rosenheck RA, Druss BG, Perlin JB. Mental disorders and quality of diabetes care in the veterans health administration. Am J Psychiatry 2002; 159: 1584–90.
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23: 934–42.
- Anderson RJ, Grigsby AB, Freedland KE, et al. Anxiety and poor glycemic control: a meta-analytic review of the literature.
Int J Psychiatry Med 2002; 32 (3): 235–47.
- McKellar JD, Humphreys K, Piette JD. Depression increases diabetes symptoms by complicating patients‘ self-care adherence. Diabetes Educ 2004; 30: 485–92.
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ.
Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001; 63: 619–30.
- Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005; 28: 2668–72.
- Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006; 333: 65.
- Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004; 363: 1589–97.
- Katon WJ, Von KM, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004; 61: 1042–49.
- Lin EH, Katon W, Rutter C, et al. Effects of enhanced depression treatment on diabetes self-care. Ann Fam Med 2006; 4: 46–53.
- Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a
double-blind, placebo-controlled trial. Psychosom Med 1997; 59:
241–50.
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind
placebo-controlled trial. Diabetes Care 2000; 23: 618–23.
- WHO. Global tuberculosis control: surveillance, planning, financing. Report WHO/HTM/TB/2005.349.. Geneva, Switzerland: World Health Organization, 2005.
- Cournos F, McKinnon K, Sullivan G. Schizophrenia and comorbid human immunodeficiency virus or hepatitis C virus.
J Clin Psychiatry 2005; 66 (suppl 6): 27–33.
- Kelly JA. HIV risk reduction interventions for persons with severe mental illness. Clin Psychol Rev 1997; 17 (3): 293–309.
- Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS 2006; 20: 731–39.
- Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs 2006; 66: 769–89.
- Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS 2006; 20: 1571–82.
- Chopra MP, Eranti SS, Chandra PS. HIV-related risk behaviors among psychiatric inpatients in India. Psychiatr Serv 1998; 49: 823–25.
- Chandra PS, Carey MP, Carey KB, Prasada Rao PS, Jairam KR, Thomas T. HIV risk behaviour among psychiatric inpatients: results from a hospital-wide screening study in southern India.
Int J STD AIDS 2003; 14: 532–38.
- McCaffery JM, Frasure-Smith N, Dube MP, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med 2006; 68: 187–200.
- Vieweg WV, Julius DA, Fernandez A, et al. Treatment of depression in patients with coronary heart disease. Am J Med 2006; 119: 567–73.
- Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001; 15: 199–226.
- Cradock-O’Leary J, Young AS, Yano EM, Wang M, Lee ML. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv 2002; 53: 874–78.
- Daumit GL, Pronovost PJ, Anthony CB, Guallar E, Steinwachs DM, Ford DE. Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Arch Gen Psychiatry 2006; 63: 267–72.
- WHO. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization, 2003.
- Weinman J, Petrie K, Moss-Morris R, Horne R. the Illness Perception Questionnaire: a new method for assesing the cognitive representation of disease. Psychol Health 1996; 11: 431–445.
- Dube B, Benton T, Cruess DG, Evans DL. Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci 2005; 30: 237–46.
- Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001; 56: 257–60.
- Neuenburg JK, Brodt HR, Herndier BG, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31: 171–77.
- White DA, Heaton RK, Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. The HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995; 1: 304–15.
- Maj M, Satz P, Janssen R, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry 1994; 51: 51–61.
- Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001; 58: 721–28.
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158: 725–30.
- Maj M, Janssen R, Starace F, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase I. Study design and psychiatric findings. Arch Gen Psychiatry 1994; 51: 39–49.
- Cohen MH, Cook JA, Grey D, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. Am J Public Health 2004; 94: 1147–51.
- Kim YJ, Peragallo N, DeForge B. Predictors of participation in an HIV risk reduction intervention for socially deprived Latino women: a cross sectional cohort study. Int J Nurs Stud 2006; 43: 527–34.
- Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA 2001; 285: 1466–74.
- Cook JA, Grey D, Burke J, et al. Depressive symptoms and
AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health 2004; 94: 1133–40.
- Evans DL, Ten Have TR, Douglas SD, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry 2002; 159: 1752–59.
- Wilkie FL, Goodkin K, Eisdorfer C, et al. Mild cognitive impairment and risk of mortality in HIV-1 infection.
J Neuropsychiatry Clin Neurosci 1998; 10 (2): 125–32.
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133: 21–30.
- Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 2004; 45: 394–402.
- Gordillo V, del AJ, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS 1999; 13: 1763–69.
- Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004; 18 (suppl 1): S19–25.
- Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology 2002; 59: 1944–50.
- Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV+ Ugandan patients purchasing therapy.
Int J STD AIDS 2005; 16: 38–41.
- Tadios Y, Davey G. Antiretroviral treatment adherence and its correlates in Addis Ababa, Ethiopia. Ethiop Med J 2006; 44: 237–44.
- Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients.
J Acquir Immune Defic Syndr 2005; 38: 432–38.
- Laperriere A, Ironson GH, Antoni MH, et al. Decreased depression up to one year following CBSM+ intervention in depressed women with AIDS: the smart/EST women’s project. J Health Psychol 2005; 10: 223–31.
- Carrico AW, Antoni MH, Pereira DB, et al. Cognitive behavioral stress management effects on mood, social support, and a marker of antiviral immunity are maintained up to 1 year in HIV-infected gay men. Int J Behav Med 2005; 12 (4): 218–26.
- Lechner SC, Antoni MH, Lydston D, et al. Cognitive-behavioral interventions improve quality of life in women with AIDS.
J Psychosom Res 2003; 54: 253–61.
- Kalichman SC, Rompa D, Cage M. Group intervention to reduce HIV transmission risk behavior among persons living with HIV/AIDS. Behav Modif 2005; 29: 256–85.
- Rabkin JG, Wagner GJ, Rabkin R. Fluoxetine treatment for depression in patients with HIV and AIDS: a randomized, placebo-controlled trial. Am J Psychiatry 1999; 156: 101–07.
- Rabkin JG, Rabkin R, Harrison W, Wagner G. Effect of imipramine on mood and enumerative measures of immune status in depressed patients with HIV illness. Am J Psychiatry 1994; 151: 516–23.
- Rabkin JG, Rabkin R, Wagner G. Effects of fluoxetine on mood and immune status in depressed patients with HIV illness.
J Clin Psychiatry 1994; 55: 92–97.
- Vitiello B, Burnam MA, Bing EG, Beckman R, Shapiro MF. Use of psychotropic medications among HIV-infected patients in the United States. Am J Psychiatry 2003; 160: 547–54.
- Chin-Hong PV, Deeks SG, Liegler T, et al. High-risk sexual behavior in adults with genotypically proven antiretroviral-resistant HIV infection. J Acquir Immune Defic Syndr 2005; 40: 463–71.
- Ohta Y, Nakane Y, Mine M, et al. The epidemiological study of physical morbidity in schizophrenics–2. Association between schizophrenia and incidence of tuberculosis. Jpn J Psychiatry Neurol 1988; 42: 41–47.
- McQuistion HL, Colson P, Yankowitz R, Susser E. Tuberculosis infection among people with severe mental illness. Psychiatr Serv 1997; 48: 833–35.
- Zeenreich A, Gochstein B, Grinshpoon A, Miron M, Rosenman J, Ben-Dov I. [Recurrent tuberculosis in a psychiatric hospital, recurrent outbreaks during 1987–1996]. Harefuah 1998; 134: 168–72, 248, 247.
- Buskin SE, Gale JL, Weiss NS, Nolan CM. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990.
Am J Public Health 1994; 84: 1750–56.
- Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 2004; 363: 474–81.
- Davidson H, Schluger NW, Feldman PH, Valentine DP, Telzak EE, Laufer FN. The effects of increasing incentives on adherence to tuberculosis directly observed therapy. Int J Tuberc Lung Dis 2000; 4: 860–65.
- Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med 1997; 102: 164–70.
- Bumburidi E, Ajeilat S, Dadu A, et al. Progress toward tuberculosis control and determinants of treatment outcomes—Kazakhstan, 2000–2002.
MMWR Morb Mortal Wkly Rep 2006; 55 (Suppl 1): 11–5.
- Shin SS, Pasechnikov AD, Gelmanova IY, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis 2006; 10: 402–08.
- Sterling TR, Zhao Z, Khan A, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors.
Int J Tuberc Lung Dis 2006; 10: 542–49.
- Tulsky JP, Hahn JA, Long HL, C et al. Can the poor adhere? Incentives for adherence to TB prevention in homeless adults. Int J Tuberc Lung Dis 2004; 8: 83–91.
- Sweetland A, Acha J, Guerra D. Enhancing adherence: the role of group psychotherapy in the treatment of MDR-TB in urban Peru. In: Cohen A, Kleinman A, Saraceno B, eds. World mental health casebook: social and mental health programmes in low-income countries. New York, USA: Kluwer Academic Press, 2002: 57–85.
- Vega P, Sweetland A, Acha J, et al. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004; 8: 749–59.
- Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry 2001; 23: 77–83.
- Aghanwa HS, Erhabor GE. Demographic/socioeconomic factors in mental disorders associated with tuberculosis in southwest Nigeria. J Psychosom Res 1998; 45: 353–60.
- Carta MG, Coppo P, Carpiniello B, Mounkuoro PP. Mental disorders and health care seeking in Bandiagara: a community survey in the Dogon Plateau. Soc Psychiatry Psychiatr Epidemiol 1997; 32: 222–29.
- Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 2006; (2): CD003343.
- Acha J, Sweetland J, Guerra D, Chalco K, Castillo H, Palacios E. Psychosocial support groups for patients with multidrug-resistant tuberculosis: five years of experience. Global Public Health (in press).
- Janmeja AK, Das SK, Bhargava R, Chavan BS. Psychotherapy improves compliance with tuberculosis treatment. Respiration 2005; 72: 375–80.
- Demissie M, Getahun H, Lindtjorn B. Community tuberculosis care through “TB clubs” in rural North Ethiopia. Soc Sci Med 2003; 56: 2009–18.
- Weiss MG. The interrelationship of tropical disease and mental disorder: conceptual framework and literature review. Part I: Malaria. Cult Med Psychiatry 1985; 9: 121–200.
- Gernaat HB. Malaria presenting as atypical depression.
Br J Psychiatry 1990; 157: 783.
- Prakash MV, Stein G. Malaria presenting as atypical depression.
Br J Psychiatry 1990; 156: 594–95.
- Osuntokun BO. Malaria and the nervous system. Afr J Med Med Sci
1983; 12: 165–72.
- Thiam MH, Diop BM, Dieng Y, Gueye M. [Mental disorders in cerebral malaria]. Dakar Med 2002; 47: 122–27.
- Lovestone S. Chloroquine-induced mania. Br J Psychiatry 1991; 159:
164–5.
- Dugbartey AT, Dugbartey MT, Apedo MY. Delayed neuropsychiatric effects of malaria in Ghana. J Nerv Ment Dis 1998; 186: 183–86.
- Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health 2006; 11: 386–97.
- Richardson ED, Varney NR, Roberts RJ, Springer JA, Wood PS. Long-term cognitive sequelae of cerebral malaria in Vietnam veterans. Appl Neuropsychol 1997; 4: 238–43.
- WHO/ UNICEF. Africa Malaria Report. Report WHO/CDS/MAL/2003.1093. Geneva, Switzerland: World Health Organization, 2003.
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004; 2: CD000363.
- Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC.
Self-reported malaria and mosquito avoidance in relation to household risk factors in a Kenyan coastal city. J Biosoc Sci 2005; 37: 761–71.
- Macintyre K, Keating J, Sosler S, et al. Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar J 2002; 1: 14.
- Adongo PB, Kirkwood B, Kendall C. How local community knowledge about malaria affects insecticide-treated net use in northern Ghana. Trop Med Int Health 2005; 10: 366–78.
- Williams HA, Jones CO. A critical review of behavioral issues related to malaria control in sub-Saharan Africa: what contributions have social scientists made? Soc Sci Med 2004; 59: 501–23.
- McCombie SC. Self-treatment for malaria: the evidence and methodological issues. Health Policy Plan 2002; 17: 333–44.
- Mwenesi HA. Social science research in malaria prevention, management and control in the last two decades: an overview. Acta Trop 2005; 95: 292–7.
- Yeung S, White NJ. How do patients use antimalarial drugs? A review of the evidence. Trop Med Int Health 2005; 10: 121–38.
- Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 2004; 364: 1896–98.
- Ray S, De Cock R, Mahari M, Chiposi ML. Clinical audit of malaria diagnosis in urban primary curative care clinics, Zimbabwe.
Cent Afr J Med 1995; 41: 385–91.
- Maier W, Gansicke M, Gater R, Rezaki M, Tiemens B, Urzua RF. Gender differences in the prevalence of depression: a survey in primary care. J Affect Disord 1999; 53: 241–52.
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations.
Acta Psychiatr Scand 2003; 108: 163–74.
- Mirza I, Jenkins R. Risk factors, prevalence, and treatment of anxiety and depressive disorders in Pakistan: systematic review. BMJ 2004; 328: 794.
- Jenkins R. Sex differences in minor psychiatric morbidity: a survey of a homogeneous population. Soc Sci Med 1985; 20: 887–99.
- Garcia-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: findings from the WHO multi-country study on women’s health and domestic violence. Lancet 2006; 368: 1260–69.
- Patel V, Kirkwood BR, Pednekar S, et al. Gender disadvantage and reproductive health risk factors for common mental disorders in women: a community survey in India. Arch Gen Psychiatry 2006; 63: 404–13.
- Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. BMJ 2006; 332: 749–55.
- Prasad J, Abraham S, Akila B, Joseph A, Jacob KS. Symptoms related to the reproductive tract and mental health among women in rural southern India. Natl Med J India 2003; 16: 303–08.
- Patel V, Pednekar S, Weiss H, et al. Why do women complain of vaginal discharge? A population survey of infectious and pyschosocial risk factors in a South Asian community.
Int J Epidemiol 2005; 34: 853–62.
- Nilsson E, Lichtenstein P, Cnattingius S, Murray RM,
Hultman CM. Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res 2002; 58: 221–29.
- Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB. Preterm birth and intra-uterine growth retardation among children of women with schizophrenia. Br J Psychiatry 1999; 175: 239–45.
- Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich LA. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry 2005; 162: 79–91.
- Webb R, Abel K, Pickles A, Appleby L. Mortality in offspring of parents with psychotic disorders: a critical review and meta-analysis. Am J Psychiatry 2005; 162: 1045–56.
- O’Hara MW. The nature of postpartum depressive disorders. In: Murray L, Cooper PJ, eds. Postpartum Depression and Child Development. New York, NY, USA: Guilford Press, 1997: 3–31.
- Murray L, Cooper PJ. Intergenerational transmission of affective and cognitive processes associated with depression: infancy and the
pre-school years. In: Goodyer IM, ed. Unipolar depression: a lifespan perspective. Oxford, UK: Oxford University Press, 2003: 17–46.
- Cooper PJ, Tomlinson M, Swartz L, Woolgar M, Murray L, Molteno C. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement.
Br J Psychiatry 1999; 175: 554–58.
- Patel V, DeSouza N, Rodrigues M. Postnatal depression and infant growth and development in low income countries: a cohort study from Goa, India. Arch Dis Child 2003; 88: 34–37.
- Ramalingaswami V, Jonsson U, Rohde J. Commentary on Nutrition, Progress of Nations. New York, NY, USA: UNICEF, 1996.
- Rahman A, Bunn J, Lovel H, Creed F. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand 2007; 115: 481–86.
- Patel V, Prince M. Maternal psychological morbidity and low birth weight in India. Br J Psychiatry 2006; 188: 284–85.
- Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am J Epidemiol 2004; 159: 872–81.
- Orr ST, James SA, Blackmore PC. Maternal prenatal depressive symptoms and spontaneous preterm births among
African-American women in Baltimore, Maryland. Am J Epidemiol
2002; 156: 797–802.
- Orr ST, James SA, Miller CA, et al. Psychosocial stressors and low birthweight in an urban population. Am J Prev Med 1996; 12: 459–66.
- Rahman A, Lovel H, Bunn J, Iqbal Z, Harrington R. Mothers’ mental health and infant growth: a case-control study from Rawalpindi, Pakistan. Child Care Health Dev 2004; 30: 21–27.
- Rahman A, Iqbal Z, Bunn J, Lovel H, Harrington R. Impact of maternal depression on infant nutritional status and illness: a cohort study. Arch Gen Psychiatry 2004; 61: 946–52.
- Anoop S, Saravanan B, Joseph A, Cherian A, Jacob KS. Maternal depression and low maternal intelligence as risk factors for malnutrition in children: a community based case-control study from South India. Arch Dis Child 2004; 89: 325–29.
- Patel V, Rahman A, Jacob KS, Hughes M. Effect of maternal mental health on infant growth in low income countries: new evidence from South Asia. BMJ 2004; 328: 820–23.
- Rahman A, Bunn J, Lovel H, Creed F. Maternal depression increases infant risk of diarrhoeal illness: a cohort study. Arch Dis Child 2007; 92: 24–28.
- Tomlinson M, Cooper PJ, Stein A, Swartz L, Molteno C.
Post-partum depression and infant growth in a South African peri-urban settlement. Child Care Health Dev 2006; 32: 81–86.
- Harpham T, Huttly S, De Silva MJ, Abramsky T. Maternal mental health and child nutritional status in four developing countries. J Epidemiol Community Health 2005; 59: 1060–64.
- Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007; 369: 145–57.
- Galler JR, Harrison RH, Ramsey F, Forde V, Butler SC. Maternal depressive symptoms affect infant cognitive development in Barbados. J Child Psychol Psychiatry 2000; 41: 747–57.
- Galler JR, Ramsey FC, Harrison RH, Taylor J, Cumberbatch G, Forde V. Postpartum maternal moods and infant size predict performance on a national high school entrance examination. J Child Psychol Psychiatry 2004; 45: 1064–75.
- Turner C, Boyle F, O’Rourke P. Mothers’ health post-partum and their patterns of seeking vaccination for their infants.
Int J Nurs Pract 2003; 9: 120–26.
- Minkovitz CS, Strobino D, Scharfstein D, et al. Maternal depressive symptoms and children’s receipt of health care in the first 3 years of life. Pediatrics 2005; 115: 306–14.
- Chung EK, McCollum KF, Elo IT, Lee HJ, Culhane JF. Maternal depressive symptoms and infant health practices among
low-income women. Pediatrics 2004; 113: 523–529.
- Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics 2006; 118: 659–68.
- Galler JR, Harrison RH, Biggs MA, Ramsey F, Forde V. Maternal moods predict breastfeeding in Barbados. J Dev Behav Pediatr 1999; 20: 80–87.
- Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med 2001; 155: 347–53.
- Patel V, Kleinman A. Poverty and common mental disorders in developing countries. Bull World Health Organ 2003; 81: 609–15.
- Edwards P, Roberts I, Green J, Lutchmun S. Deaths from injury in children and employment status in family: analysis of trends in class specific death rates. BMJ 2006; 333: 119.
- Peden M, Scurfield R, Sleet D, et al. Summary: world report on road traffic injury prevention. Geneva, Switzerland: World Health Organization, 2004.
- Odero W, Garner P, Zwi A. Road traffic injuries in developing countries: a comprehensive review of epidemiological studies. Trop Med Int Health 1997; 2: 445–60.
- Nantulya VM, Reich MR. Equity dimensions of road traffic injuries in low- and middle-income countries. Inj Control Saf Promot 2003; 10: 13–20.
- Borkenstein RF, Crowther RF, Shumate RP, Ziel WB, Zylman R. The role of the drinking driver in traffic crashes.Bloomington, Indiana, USA: Department of Police Administration, Indiana University, 1964.
- Wang Z, Jiang J. An overview of research advances in road traffic trauma in China. Traffic Inj Prev 2003; 4: 9–16.
- Grossman DC, Soderberg R, Rivara FP. Prior injury and motor vehicle crash as risk factors for youth suicide. Epidemiology 1993; 4: 115–19.
- Sibert R. Stress in families of children who have ingested poisons.
BMJ 1975; 3: 87–89.
- Brown GW, Davidson S. Social class, psychiatric disorder of mother, and accidents to children. Lancet 1978; 1: 378–81.
- O’Connor TG, Davies L, Dunn J, Golding J. Distribution of accidents, injuries, and illnesses by family type. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Pediatrics 2000; 106: e68.
- Howe LD, Huttly SR, Abramsky T. Risk factors for injuries in young children in four developing countries: the Young Lives Study.
Trop Med Int Health 2006; 11: 1557–66.
- McLennan JD, Kotelchuck M. Parental prevention practices for young children in the context of maternal depression. Pediatrics 2000; 105: 1090–95.
- Leiferman J. The effect of maternal depressive symptomatology on maternal behaviors associated with child health. Health Educ Behav 2002; 29: 596–607.
- Mulvaney C, Kendrick D. Do maternal depressive symptoms, stress and a lack of social support influence whether mothers living in deprived circumstances adopt safety practices for the prevention of childhood injury? Child Care Health Dev 2006; 32: 311–19.
- Li L, Roberts I, Power C. Physical and psychological effects of injury. Data from the 1958 British birth cohort study.
Eur J Public Health 2001; 11: 81–83.
- Stoddard FJ, Ronfeldt H, Kagan J, et al. Young burned children: the course of acute stress and physiological and behavioral responses. Am J Psychiatry 2006; 163: 1084–90.
- WHO. World report on violence and health. Geneva, Switzerland: World Health Organization; 2002.
- Mollica RF, Cardozo BL, Osofsky HJ, Raphael B, Ager A, Salama P. Mental health in complex emergencies. Lancet 2004; 364: 2058–67.
- de Jong JT, Komproe IH, Van Ommeren M. Common mental disorders in postconflict settings. Lancet 2003; 361: 2128–30.
- Eytan A, Gex-Fabry M, Toscani L, Deroo L, Loutan L, Bovier PA. Determinants of postconflict symptoms in Albanian Kosovars. J Nerv Ment Dis 2004; 192: 664–71.
- Scholte WF, Olff M, Ventevogel P, et al. Mental health symptoms following war and repression in eastern Afghanistan. JAMA 2004; 292: 585–93.
- Marshall GN, Schell TL, Elliott MN, Berthold SM, Chun CA. Mental health of Cambodian refugees 2 decades after resettlement in the United States. JAMA 2005; 294: 571–79.
- Sabin M, Lopes CB, Nackerud L, Kaiser R, Varese L. Factors associated with poor mental health among Guatemalan refugees living in Mexico 20 years after civil conflict. JAMA 2003; 290: 635–42.
- Patel V, Saxena S, Thornicroft G, et al. Scale up services for mental disorders: a call for action. Lancet 2007; published online Sept 4. DOI:10.1016/S0140-6736(07)61242-2.
- Saxena S, Thornicroft G, Knapp, M Whiteford, H. Resources for mental health: scarcity, inequity, and inefficiency. Lancet 2007; published online Sept 4. DOI:10.1016/S0140-6736(07)61239-2.
- Epping-Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of health care for chronic conditions. Qual Saf Health Care 2004; 13: 299–305.
- Patel V, Araya R, Chatterjee S, et al. Treatment and prevention of mental disorders in low-income and middle-income countries Lancet 2007; published online Sept 4. DOI:10.1016/S0140- 6736(07)61240-9.
- Bolton P, Bass J, Neugebauer R, et al. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA 2003; 289: 3117–24.
- Patel V, Chisholm D, Rabe-Hesketh S, Dias-Saxena F, Andrew G, Mann A. Efficacy and cost-effectiveness of drug and psychological treatments for common mental disorders in general health care in Goa, India: a randomised, controlled trial. Lancet 2003; 361: 33–39.
- Araya R, Rojas G, Fritsch R, et al. Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 2003; 361: 995–1000.
- Narayan KM, Thompson TJ, Boyle JP, et al. The use of population attributable risk to estimate the impact of prevention and early detection of type 2 diabetes on population-wide mortality risk in US males. Health Care Manag Sci 1999; 2: 223–27.
- Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet 2007; 369: 60–70.
- Shi QC, Zhang JM, Xu FZ, et al. [Epidemiological survey of mental illnesses in the people aged 15 and older in Zhejiang Province, China]. Zhonghua Yu Fang Yi Xue Za Zhi 2005; 39: 229–36.
- Wang H, Mullahy J. Willingness to pay for reducing fatal risk by improving air quality: a contingent valuation study in Chongqing, China. Sci Total Environ 2006; 367: 50–57.
- Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med 2006; 36: 13–34.
- Patel V, Saraceno B, Kleinman A. Beyond evidence: the moral case for international mental health. Am J Psychiatry 2006; 163: 1312–15.
